Direct endothelial junction restoration results in significant tumor vascular normalization and metastasis inhibition in mice
- PMID: 24811731
- PMCID: PMC4058043
- DOI: 10.18632/oncotarget.1942
Direct endothelial junction restoration results in significant tumor vascular normalization and metastasis inhibition in mice
Abstract
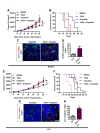
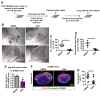
Tumor blood vessels are leaky and immature, which causes inadequate blood supply to tumor tissues resulting in hypoxic microenvironment and promotes metastasis. Here we have explored tumor vessel modulating activity of Sac-1004, a recently developed molecule in our lab, which directly potentiates VE-cadherin-mediated endothelial cell junction. Sac-1004 could enhance vascular junction integrity in tumor vessels and thereby inhibit vascular leakage and enhance vascular perfusion. Improved perfusion enabled Sac-1004 to have synergistic anti-tumor effect on cisplatin-mediated apoptosis of tumor cells. Interestingly, characteristics of normalized blood vessels namely reduced hypoxia, improved pericyte coverage and decreased basement membrane thickness were readily observed in tumors treated with Sac-1004. Remarkably, Sac-1004 was also able to inhibit lung and lymph node metastasis in MMTV and B16BL6 tumor models. This was in correlation with a reduction in epithelial-to-mesenchymal transition of tumor cells with considerable diminution in expression of related transcription factors. Moreover, cancer stem cell population dropped substantially in Sac-1004 treated tumor tissues. Taken together, our results showed that direct restoration of vascular junction could be a significant strategy to induce normalization of tumor blood vessels and reduce metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. - PubMed
-
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–1370. - PubMed
-
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

