FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway
- PMID: 24811863
- PMCID: PMC4058020
- DOI: 10.18632/oncotarget.1723
FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway
Abstract
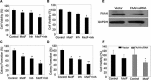
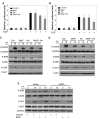
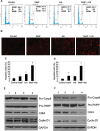
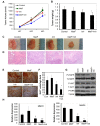
The endocannabinoid anandamide (AEA), a neurotransmitter was shown to have anti-cancer effects. Fatty acid amide hydrolase (FAAH) metabolizes AEA and decreases its anti-tumorigenic activity. In this study, we have analyzed the role of FAAH inhibition in non-small cell lung cancer (NSCLC). We have shown that FAAH and CB1 receptor which is activated by AEA are expressed in lung adenocarcinoma patient samples and NSCLC cell lines A549 and H460. Since the synthetic analogue of anandamide (Met-F-AEA) did not possess significant anti-tumorigenic effects, we used Met-F-AEA in combination with FAAH inhibitor URB597 which significantly reduced EGF (epidermal growth factor)-induced proliferative and chemotactic activities in vitro when compared to anti-tumorigenic activity of Met-F-AEA alone. Further analysis of signaling mechanisms revealed that Met-F-AEA in combination with URB597 inhibits activation of EGFR and its downstream signaling ERK, AKT and NF-kB. In addition, it inhibited MMP2 secretion and stress fiber formation. We have also shown that the Met-F-AEA in combination with URB597 induces G0/G1 cell cycle arrest by downregulating cyclin D1 and CDK4 expressions, ultimately leading to apoptosis via activation of caspase-9 and PARP. Furthermore, the combination treatment inhibited tumor growth in a xenograft nude mouse model system. Tumors derived from Met-F-AEA and URB597 combination treated mice showed reduced EGFR, AKT and ERK activation and MMP2/MMP9 expressions when compared to Met-F-AEA or URB597 alone. Taken together, these data suggest in EGFR overexpressing NSCLC that the combination of Met-F-AEA with FAAH inhibitor resulted in superior therapeutic response compared to individual compound activity alone.
Conflict of interest statement
The authors disclose no competing interests
Figures


Similar articles
-
Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells.Oncotarget. 2016 Mar 22;7(12):15047-64. doi: 10.18632/oncotarget.7592. Oncotarget. 2016. PMID: 26930716 Free PMC article.
-
Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model.Mol Cell Biochem. 2019 Mar;453(1-2):143-155. doi: 10.1007/s11010-018-3439-0. Epub 2018 Aug 29. Mol Cell Biochem. 2019. PMID: 30159798
-
Fatty acid amide hydrolase determines anandamide-induced cell death in the liver.J Biol Chem. 2006 Apr 14;281(15):10431-8. doi: 10.1074/jbc.M509706200. Epub 2006 Jan 17. J Biol Chem. 2006. PMID: 16418162
-
Amygdala FAAH and anandamide: mediating protection and recovery from stress.Trends Pharmacol Sci. 2013 Nov;34(11):637-44. doi: 10.1016/j.tips.2013.08.008. Epub 2013 Oct 25. Trends Pharmacol Sci. 2013. PMID: 24325918 Free PMC article. Review.
-
The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence.Life Sci. 2013 Mar 19;92(8-9):458-62. doi: 10.1016/j.lfs.2012.05.015. Epub 2012 Jun 12. Life Sci. 2013. PMID: 22705310 Free PMC article. Review.
Cited by
-
Targeting Fatty Acid Amide Hydrolase Counteracts the Epithelial-to-Mesenchymal Transition in Keratinocyte-Derived Tumors.Int J Mol Sci. 2023 Dec 12;24(24):17379. doi: 10.3390/ijms242417379. Int J Mol Sci. 2023. PMID: 38139209 Free PMC article.
-
Endocannabinoid System and Tumour Microenvironment: New Intertwined Connections for Anticancer Approaches.Cells. 2021 Dec 2;10(12):3396. doi: 10.3390/cells10123396. Cells. 2021. PMID: 34943903 Free PMC article. Review.
-
Morphine Analgesia, Cannabinoid Receptor 2, and Opioid Growth Factor Receptor Cancer Tissue Expression Improve Survival after Pancreatic Cancer Surgery.Cancers (Basel). 2023 Aug 9;15(16):4038. doi: 10.3390/cancers15164038. Cancers (Basel). 2023. PMID: 37627066 Free PMC article.
-
N-linoleyltyrosine resisted the growth of non-small cell lung cancer cells via the regulation of CB1 and CB2 involvement of PI3K and ERK pathways.Front Pharmacol. 2023 Jun 8;14:1164367. doi: 10.3389/fphar.2023.1164367. eCollection 2023. Front Pharmacol. 2023. PMID: 37361232 Free PMC article.
-
Molecular Mechanism of Cannabinoids in Cancer Progression.Int J Mol Sci. 2021 Apr 1;22(7):3680. doi: 10.3390/ijms22073680. Int J Mol Sci. 2021. PMID: 33916164 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Salgia R, Hensing T, Campbell N, Salama AK, Maitland M, Hoffman P, Villaflor V, Vokes EE. Personalized treatment of lung cancer. Semin Oncol. 2011;38(2):274–283. - PubMed
-
- Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3(10):745–755. - PubMed
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

