Use of non-assigned interventions in a randomized trial of internet and telephone treatment for smoking cessation
- PMID: 24812022
- PMCID: PMC4207870
- DOI: 10.1093/ntr/ntu066
Use of non-assigned interventions in a randomized trial of internet and telephone treatment for smoking cessation
Abstract
Introduction: A recent meta-analysis of Internet interventions for smoking cessation found mixed evidence regarding effectiveness. One explanation may be differential use of non-assigned cessation treatments-including other Internet programs-that either amplify or mask study intervention effects. We examined the impact of non-assigned treatment use on cessation outcomes in The iQUITT Study, a randomized trial of Internet and telephone treatment for smoking cessation.
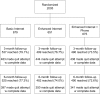
Methods: Participants were randomized to a basic Internet (BI) comparison condition (N = 675), enhanced Internet (EI: N = 651), or EI plus telephone counseling (EI+P: N = 679). The primary outcome was 30-day point prevalence abstinence (ppa) at 3 and 6 months. Assigned intervention use was assessed with automated tracking data. Assessment of non-assigned treatments included pharmacotherapy, behavioral, alternative, and non-study Internet treatments. Univariate and multivariate logistic regression models examined whether non-assigned treatment use was associated with 30-day ppa.
Results: About 70% of participants used at least one non-assigned treatment. A higher rate of non-study Internet treatment among BI participants was the only treatment group difference at both 3 and 6 months. Multivariate models controlling for condition and baseline predictors of non-assigned treatment use showed that high-intensity non-study Internet treatment was positively associated with 30-day ppa at 3 and 6 months, and pharmacotherapy and behavioral treatment use was negatively associated with 30-day ppa at 6 months.
Conclusions: Non-assigned treatment use is an important factor to consider when evaluating Internet cessation interventions. Results highlight methodological issues in selecting a comparison condition. Researchers should report non-assigned treatment use alongside main trial outcomes.
Trial registration: ClinicalTrials.gov NCT00282009.
© The Author 2014. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Andresen E. M., Malmgren J. A., Carter W. B., Patrick D. L. (1994). Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). American Journal of Preventive Medicine, 10, 77–84. - PubMed
-
- Bennett G. G., Glasgow R. E. (2009). The delivery of public health interventions via the Internet: Actualizing their potential. Annual Review of Public Health, 30, 273–292. 10.1146/annurev.publhealth.031308.100235 - PubMed
-
- Bock B., Graham A., Sciamanna C., Krishnamoorthy J., Whiteley J., Carmona-Barros R., … Abrams D. (2004). Smoking cessation treatment on the Internet: Content, quality, and usability. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 6, 207–219. 10.1080/14622200410001676332 - PubMed
-
- Bock B.C., Graham A.L., Cartter C., Cobb N. (2002, February 20–24). Smoking Cessation on the Web: Initial Evaluation of QuitNet. Paper presented at the Society for Research on Nicotine and Tobacco, Savannah, GA.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical