Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma
- PMID: 24812408
- PMCID: PMC4079734
- DOI: 10.1158/1078-0432.CCR-13-2797
Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma
Abstract
Purpose: PD-L1 is the main ligand for the immune inhibitory receptor PD-1. This ligand is frequently expressed by melanoma cells. In this study, we investigated whether PD-L1 expression is controlled by melanoma driver mutations and modified by oncogenic signaling inhibition.
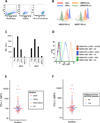
Experimental design: Expression of PD-L1 was investigated in a panel of 51 melanoma cell lines containing different oncogenic mutations, including cell lines with innate and acquired resistance to BRAF inhibitors (BRAFi). The effects of targeted therapy drugs on expression of PD-L1 by melanoma cells were investigated.
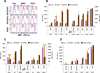
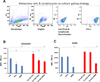
Results: No association was found between the level of PD-L1 expression and mutations in BRAF, NRAS, PTEN, or amplification of AKT. Resistance to vemurafenib due to the activation of alternative signaling pathways was accompanied with the induction of PD-L1 expression, whereas the resistance due to the reactivation of the MAPK pathway had no effect on PD-L1 expression. In melanoma cell lines, the effects of BRAF, MEK, and PI3K inhibitors on expression of PD-L1 were variable from reduction to induction, particularly in the presence of INFγ. In PD-L1-exposed lymphocytes, vemurafenib paradoxically restored activity of the MAPK pathway and increased the secretion of cytokines.
Conclusions: In melanoma cell lines, including BRAFi-resistant cells, PD-L1 expression is variably regulated by oncogenic signaling pathways. PD-L1-exposed lymphocytes decrease MAPK signaling, which is corrected by exposure to vemurafenib, providing potential benefits of combining this drug with immunotherapies.
©2014 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Alcalá AM, Flaherty KT. BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res. 2012 Jan 1;18(1):33–39. - PubMed
-
- Robert C, Thomas L, Bondarenko I, O'Day S, MD JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011 Jun 30;364(26):2517–2526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

