Early Trypanosoma cruzi infection reprograms human epithelial cells
- PMID: 24812617
- PMCID: PMC4000934
- DOI: 10.1155/2014/439501
Early Trypanosoma cruzi infection reprograms human epithelial cells
Abstract
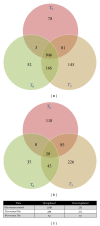
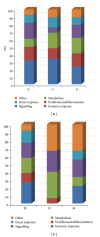
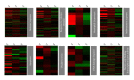
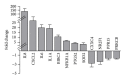
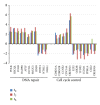
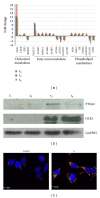
Trypanosoma cruzi, the causative agent of Chagas disease, has the peculiarity, when compared with other intracellular parasites, that it is able to invade almost any type of cell. This property makes Chagas a complex parasitic disease in terms of prophylaxis and therapeutics. The identification of key host cellular factors that play a role in the T. cruzi invasion is important for the understanding of disease pathogenesis. In Chagas disease, most of the focus is on the response of macrophages and cardiomyocytes, since they are responsible for host defenses and cardiac lesions, respectively. In the present work, we studied the early response to infection of T. cruzi in human epithelial cells, which constitute the first barrier for establishment of infection. These studies identified up to 1700 significantly altered genes regulated by the immediate infection. The global analysis indicates that cells are literally reprogrammed by T. cruzi, which affects cellular stress responses (neutrophil chemotaxis, DNA damage response), a great number of transcription factors (including the majority of NF κ B family members), and host metabolism (cholesterol, fatty acids, and phospholipids). These results raise the possibility that early host cell reprogramming is exploited by the parasite to establish the initial infection and posterior systemic dissemination.
Figures

References
-
- Maeda FY, Cortez C, Alves RM, Yoshida N. Mammalian cell invasion by closely related Trypanosoma species T. dionisii and T. cruzi . Acta Tropica. 2012;121(2):141–147. - PubMed
-
- El-Sayed NM, et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309(5733):409–415. - PubMed
-
- Atwood JA, III, Weatherly DB, Minning TA, et al. Microbiology: the Trypanosoma cruzi proteome. Science. 2005;309(5733):473–476. - PubMed
-
- Caradonna KL, Burleigh BA. Mechanisms of host cell invasion by Trypanosoma cruzi . Advances in Parasitology. 2011;76:33–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

