Antiphospholipase A₂ receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy
- PMID: 24812637
- PMCID: PMC4000632
- DOI: 10.1155/2014/143274
Antiphospholipase A₂ receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy
Abstract
Background: The recent identification of circulating autoantibodies directed towards the M-type phospholipase A2 receptor (PLA2R) has been a major advancement in the serological diagnosis of idiopathic membranous nephropathy (IMN), a common cause of nephrotic syndrome in adults. The goal of this study was to compare the performance characteristics of two commercial assays as well as the first addressable laser bead immunoassay (ALBIA) developed for the detection of anti-PLA2R antibodies.
Methods: Serum samples of 157 IMN patients and 142 controls were studied. Samples were tested by a cell based immunofluorescence assay (CBA-IFA, Euroimmun, Germany), by ELISA (Euroimmun), and by a novel ALBIA employing an in vivo expressed recombinant human PLA2R.
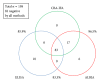
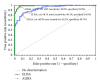
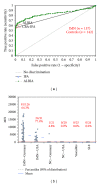
Results: Overall, the three assays showed significant qualitative and quantitative correlation. As revealed by receiver operating characteristic analysis, the ALBIA correlated better with the CBA-IFA than the ELISA (P = 0.0003). The clinical sensitivities/specificities for IMN were 60.0% (51.0-68.5%)/98.6% (95.0-99.8%) and 56.2% (47.2-64.8%)/100.0% (97.4-100.0%) for ALBIA and CBA-IFA, respectively.
Conclusion: The ALBIA represents a promising assay for the detection of anti-PLA2R antibodies showing similar performance to the CBA-IFA and the advantage of ease of use and suitability for high throughput, rapid turnaround times, and multiplexing.
Figures
References
-
- Debiec H, Martin L, Jouanneau C, et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and de novo membranous nephropathy. American Journal of Transplantation. 2011;11(10):2144–2152. - PubMed
-
- Hofstra JM, Wetzels JF. Anti-PLA2R antibodies in membranous nephropathy: ready for routine clinical practice? Netherlands Journal of Medicine. 2012;70(3):109–113. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

