A proliferative burst during preadolescence establishes the final cardiomyocyte number
- PMID: 24813607
- PMCID: PMC4078902
- DOI: 10.1016/j.cell.2014.03.035
A proliferative burst during preadolescence establishes the final cardiomyocyte number
Abstract
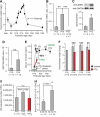
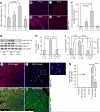
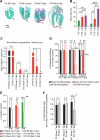
It is widely believed that perinatal cardiomyocyte terminal differentiation blocks cytokinesis, thereby causing binucleation and limiting regenerative repair after injury. This suggests that heart growth should occur entirely by cardiomyocyte hypertrophy during preadolescence when, in mice, cardiac mass increases many-fold over a few weeks. Here, we show that a thyroid hormone surge activates the IGF-1/IGF-1-R/Akt pathway on postnatal day 15 and initiates a brief but intense proliferative burst of predominantly binuclear cardiomyocytes. This proliferation increases cardiomyocyte numbers by ~40%, causing a major disparity between heart and cardiomyocyte growth. Also, the response to cardiac injury at postnatal day 15 is intermediate between that observed at postnatal days 2 and 21, further suggesting persistence of cardiomyocyte proliferative capacity beyond the perinatal period. If replicated in humans, this may allow novel regenerative therapies for heart diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
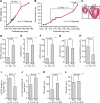
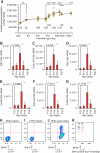
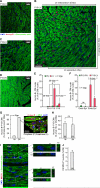
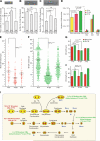
Comment in
-
Proliferation at the heart of preadolescence.Cell. 2014 May 8;157(4):765-7. doi: 10.1016/j.cell.2014.04.025. Cell. 2014. PMID: 24813600
-
Muscling up the heart: a preadolescent cardiomyocyte proliferation contributes to heart growth.Circ Res. 2014 Sep 26;115(8):690-2. doi: 10.1161/CIRCRESAHA.114.304632. Circ Res. 2014. PMID: 25258401 Free PMC article.
-
Cardiomyocyte Cell-Cycle Activity during Preadolescence.Cell. 2015 Nov 5;163(4):781-2. doi: 10.1016/j.cell.2015.10.037. Cell. 2015. PMID: 26544927 No abstract available.
-
Cardiomyocytes Replicate and their Numbers Increase in Young Hearts.Cell. 2015 Nov 5;163(4):783-4. doi: 10.1016/j.cell.2015.10.038. Cell. 2015. PMID: 26544928 Free PMC article. No abstract available.
-
No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice.Cell. 2015 Nov 5;163(4):1026-36. doi: 10.1016/j.cell.2015.10.035. Cell. 2015. PMID: 26544945
References
-
- Bizzozero G. Growth and regeneration of the organism. Br. Med. J. 1894;1:728–732.
-
- Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 2004;279:35858–35866. - PubMed
-
- Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J. Thorac. Cardiovasc. Surg. 2001;122:1023–1025. - PubMed
-
- Dai W, Kloner RA. Cardioprotection of insulin-like growth factor-1 during reperfusion therapy: what is the underlying mechanism or mechanisms? Circ Cardiovasc Interv. 2011;4:311–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

