A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response
- PMID: 24813610
- PMCID: PMC4038154
- DOI: 10.1016/j.cell.2014.03.040
A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response
Abstract
Fragile X syndrome, a common form of inherited intellectual disability, is caused by loss of the fragile X mental retardation protein FMRP. FMRP is present predominantly in the cytoplasm, where it regulates translation of proteins that are important for synaptic function. We identify FMRP as a chromatin-binding protein that functions in the DNA damage response (DDR). Specifically, we show that FMRP binds chromatin through its tandem Tudor (Agenet) domain in vitro and associates with chromatin in vivo. We also demonstrate that FMRP participates in the DDR in a chromatin-binding-dependent manner. The DDR machinery is known to play important roles in developmental processes such as gametogenesis. We show that FMRP occupies meiotic chromosomes and regulates the dynamics of the DDR machinery during mouse spermatogenesis. These findings suggest that nuclear FMRP regulates genomic stability at the chromatin interface and may impact gametogenesis and some developmental aspects of fragile X syndrome.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Y.S. is a co-founder of Constellation Pharmaceuticals and a member of its advisory board.
Figures

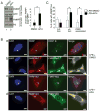
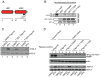
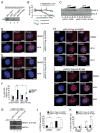


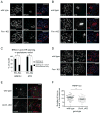
Comment in
-
FMRP: a new chapter with chromatin.Protein Cell. 2014 Dec;5(12):885-8. doi: 10.1007/s13238-014-0105-5. Protein Cell. 2014. PMID: 25327144 Free PMC article. No abstract available.
References
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
-
- Blonden L, van’t Padje S, Severijnen LA, Destree O, Oostra BA, Willemsen R. Two members of the Fxr gene family, Fmr1 and Fxr1, are differentially expressed in Xenopus tropicalis. Int J Dev Biol. 2005;49:437–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD075591/HD/NICHD NIH HHS/United States
- AG00222-7/AG/NIA NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- R01 CA118487/CA/NCI NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- MH096066/MH/NIMH NIH HHS/United States
- R01 MH096066/MH/NIMH NIH HHS/United States
- 092809/Wellcome Trust/United Kingdom
- R01 HD020521/HD/NICHD NIH HHS/United States
- R01 MH080129/MH/NIMH NIH HHS/United States
- R01 GM043901/GM/NIGMS NIH HHS/United States
- R37 HD020521/HD/NICHD NIH HHS/United States
- F32 HD075591/HD/NICHD NIH HHS/United States
- R01 GM101664/GM/NIGMS NIH HHS/United States
- NCI118487/PHS HHS/United States
- T32 AG000222/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HD024064/HD/NICHD NIH HHS/United States
- HD020521/HD/NICHD NIH HHS/United States
- R01 MH102690/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

