GroEL/ES chaperonin modulates the mechanism and accelerates the rate of TIM-barrel domain folding
- PMID: 24813614
- PMCID: PMC4071350
- DOI: 10.1016/j.cell.2014.03.038
GroEL/ES chaperonin modulates the mechanism and accelerates the rate of TIM-barrel domain folding
Abstract
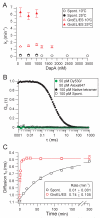
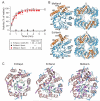
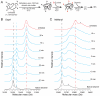
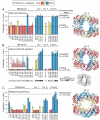
The GroEL/ES chaperonin system functions as a protein folding cage. Many obligate substrates of GroEL share the (βα)8 TIM-barrel fold, but how the chaperonin promotes folding of these proteins is not known. Here, we analyzed the folding of DapA at peptide resolution using hydrogen/deuterium exchange and mass spectrometry. During spontaneous folding, all elements of the DapA TIM barrel acquire structure simultaneously in a process associated with a long search time. In contrast, GroEL/ES accelerates folding more than 30-fold by catalyzing segmental structure formation in the TIM barrel. Segmental structure formation is also observed during the fast spontaneous folding of a structural homolog of DapA from a bacterium that lacks GroEL/ES. Thus, chaperonin independence correlates with folding properties otherwise enforced by protein confinement in the GroEL/ES cage. We suggest that folding catalysis by GroEL/ES is required by a set of proteins to reach native state at a biologically relevant timescale, avoiding aggregation or degradation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Azia A, Unger R, Horovitz A. What distinguishes GroEL substrates from other Escherichia coli proteins? FEBS J. 2012;279:543–550. - PubMed
-
- Baumketner A, Jewett A, Shea JE. Effects of confinement in chaperonin assisted protein folding: Rate enhancement by decreasing the roughness of the folding energy landscape. J. Mol. Biol. 2003;332:701–713. - PubMed
-
- Brinker A, Pfeifer G, Kerner MJ, Naylor DJ, Hartl FU, Hayer-Hartl M. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 2001;107:223–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

