Transit-amplifying cells orchestrate stem cell activity and tissue regeneration
- PMID: 24813615
- PMCID: PMC4041217
- DOI: 10.1016/j.cell.2014.02.057
Transit-amplifying cells orchestrate stem cell activity and tissue regeneration
Abstract
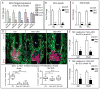
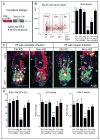
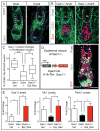
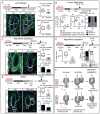
Transit-amplifying cells (TACs) are an early intermediate in tissue regeneration. Here, using hair follicles (HFs) as a paradigm, we show that emerging TACs constitute a signaling center that orchestrates tissue growth. Whereas primed stem cells (SCs) generate TACs, quiescent SCs only proliferate after TACs form and begin expressing Sonic Hedgehog (SHH). TAC generation is independent of autocrine SHH, but the TAC pool wanes if they can't produce SHH. We trace this paradox to two direct actions of SHH: promoting quiescent-SC proliferation and regulating dermal factors that stoke TAC expansion. Ingrained within quiescent SCs' special sensitivity to SHH signaling is their high expression of GAS1. Without sufficient input from quiescent SCs, replenishment of primed SCs for the next hair cycle is compromised, delaying regeneration and eventually leading to regeneration failure. Our findings unveil TACs as transient but indispensable integrators of SC niche components and reveal an intriguing interdependency of primed and quiescent SC populations on tissue regeneration.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Tic-TACs: refreshing hair growth.Cell. 2014 May 8;157(4):769-70. doi: 10.1016/j.cell.2014.04.014. Cell. 2014. PMID: 24813602 Free PMC article.
References
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

