Outcomes in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with isolated deletion 5q treated with lenalidomide: a subset analysis from the MDS-004 study
- PMID: 24813620
- PMCID: PMC4232868
- DOI: 10.1111/ejh.12380
Outcomes in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with isolated deletion 5q treated with lenalidomide: a subset analysis from the MDS-004 study
Abstract
Objective: A subset analysis of the randomised, phase 3, MDS-004 study to evaluate outcomes in patients with International Prognostic Scoring System (IPSS)-defined Low-/Intermediate (Int)-1-risk myelodysplastic syndromes (MDS) with isolated del(5q).
Methods: Patients received lenalidomide 10 mg/d (days 1-21; n = 47) or 5 mg/d (days 1-28; n = 43) on 28-d cycles or placebo (n = 45). From the placebo and lenalidomide 5 mg groups, 84% and 58% of patients, respectively, crossed over to lenalidomide 5 or 10 mg at 16 wk, respectively.
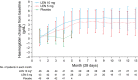
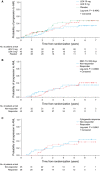
Results: Rates of red blood cell-transfusion independence (RBC-TI) ≥182 d were higher in the lenalidomide 10 mg (57.4%; P < 0.0001) and 5 mg (37.2%; P = 0.0001) groups vs. placebo (2.2%). Cytogenetic response rates (major + minor responses) were 56.8% (P < 0.0001), 23.1% (P = 0.0299) and 0%, respectively. Two-year cumulative risk of acute myeloid leukaemia progression was 12.6%, 17.4% and 16.7% in the lenalidomide 10 mg, 5 mg, and placebo groups, respectively. In a 6-month landmark analysis, overall survival was longer in lenalidomide-treated patients with RBC-TI ≥182 d vs. non-responders (P = 0.0072). The most common grade 3-4 adverse event was myelosuppression.
Conclusions: These data support the clinical benefits and acceptable safety profile of lenalidomide in transfusion-dependent patients with IPSS-defined Low-/Int-1-risk MDS with isolated del(5q).
Keywords: acute myeloid leukaemia; del(5q); lenalidomide; myelodysplastic syndromes; transfusion independence.
© 2014 The Authors. European Journal of Haematology Published by John Wiley & Sons Ltd.
Figures

References
-
- Bernasconi P, Klersy C, Boni M, et al. World Health Organization classification in combination with cytogenetic markers improves the prognostic stratification of patients with de novo primary myelodysplastic syndromes. Br J Haematol. 2007;137:193–205. - PubMed
-
- Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–95. - PubMed
-
- Solé F, Luño E, Sanzo C, et al. Identification of novel cytogenetic markers with prognostic significance in a series of 968 patients with primary myelodysplastic syndromes. Haematologica. 2005;90:1168–78. - PubMed
-
- Germing U, Lauseker M, Hildebrandt B, et al. Survival, prognostic factors, and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): a multicenter study. Leukemia. 2012;26:1286–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

