Nonmuscle myosin II isoforms coassemble in living cells
- PMID: 24814144
- PMCID: PMC4108432
- DOI: 10.1016/j.cub.2014.03.071
Nonmuscle myosin II isoforms coassemble in living cells
Erratum in
- Curr Biol. 2015 Feb 2;25(3):402
-
Nonmuscle Myosin II Isoforms Coassemble in Living Cells.Curr Biol. 2015 Feb 2;25(3):402. doi: 10.1016/j.cub.2015.01.028. Epub 2015 Feb 2. Curr Biol. 2015. PMID: 29665398 No abstract available.
Abstract
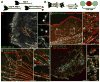
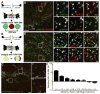
Nonmuscle myosin II (NM II) powers myriad developmental and cellular processes, including embryogenesis, cell migration, and cytokinesis [1]. To exert its functions, monomers of NM II assemble into bipolar filaments that produce a contractile force on the actin cytoskeleton. Mammalian cells express up to three isoforms of NM II (NM IIA, IIB, and IIC), each of which possesses distinct biophysical properties and supports unique as well as redundant cellular functions [2-8]. Despite previous efforts [9-13], it remains unclear whether NM II isoforms assemble in living cells to produce mixed (heterotypic) bipolar filaments or whether filaments consist entirely of a single isoform (homotypic). We addressed this question using fluorescently tagged versions of NM IIA, IIB, and IIC, isoform-specific immunostaining of the endogenous proteins, and two-color total internal reflection fluorescence structured-illumination microscopy, or TIRF-SIM, to visualize individual myosin II bipolar filaments inside cells. We show that NM II isoforms coassemble into heterotypic filaments in a variety of settings, including various types of stress fibers, individual filaments throughout the cell, and the contractile ring. We also show that the differential distribution of NM IIA and NM IIB typically seen in confocal micrographs of well-polarized cells is reflected in the composition of individual bipolar filaments. Interestingly, this differential distribution is less pronounced in freshly spread cells, arguing for the existence of a sorting mechanism acting over time. Together, our work argues that individual NM II isoforms are potentially performing both isoform-specific and isoform-redundant functions while coassembled with other NM II isoforms.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006;281:35873–35883. - PubMed
-
- Kim KY, Kovacs M, Kawamoto S, Sellers JR, Adelstein RS. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem. 2005;280:22769–22775. - PubMed
-
- Bao J, Ma X, Liu C, Adelstein RS. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem. 2007;282:22102–22111. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

