NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease
- PMID: 24814483
- PMCID: PMC4051987
- DOI: 10.1016/j.cmet.2014.04.001
NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease
Abstract
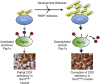
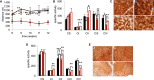
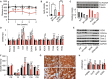
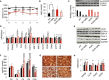
Mitochondrial disorders are highly heterogeneous conditions characterized by defects of the mitochondrial respiratory chain. Pharmacological activation of mitochondrial biogenesis has been proposed as an effective means to correct the biochemical defects and ameliorate the clinical phenotype in these severely disabling, often fatal, disorders. Pathways related to mitochondrial biogenesis are targets of Sirtuin1, a NAD(+)-dependent protein deacetylase. As NAD(+) boosts the activity of Sirtuin1 and other sirtuins, intracellular levels of NAD(+) play a key role in the homeostatic control of mitochondrial function by the metabolic status of the cell. We show here that supplementation with nicotinamide riboside, a natural NAD(+) precursor, or reduction of NAD(+) consumption by inhibiting the poly(ADP-ribose) polymerases, leads to marked improvement of the respiratory chain defect and exercise intolerance of the Sco2 knockout/knockin mouse, a mitochondrial disease model characterized by impaired cytochrome c oxidase biogenesis. This strategy is potentially translatable into therapy of mitochondrial disorders in humans.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Adamietz P. Poly(ADP-ribose) synthase is the major endogenous nonhistone acceptor for poly(ADP-ribose) in alkylated rat hepatoma cells. Eur. J. Biochem. 1987;169:365–372. - PubMed
-
- Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. - PubMed
-
- Bugiani M., Invernizzi F., Alberio S., Briem E., Lamantea E., Carrara F., Moroni I., Farina L., Spada M., Donati M.A. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta. 2004;1659:136–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

