Alternative chelator for ⁸⁹Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO)
- PMID: 24814511
- PMCID: PMC4059252
- DOI: 10.1021/jm500389b
Alternative chelator for ⁸⁹Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO)
Abstract
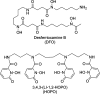
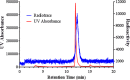
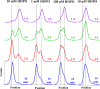
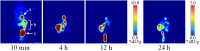
Zirconium-89 is an effective radionuclide for antibody-based positron emission tomography (PET) imaging because its physical half-life (78.41 h) matches the biological half-life of IgG antibodies. Desferrioxamine (DFO) is currently the preferred chelator for (89)Zr(4+); however, accumulation of (89)Zr in the bones of mice suggests that (89)Zr(4+) is released from DFO in vivo. An improved chelator for (89)Zr(4+) could eliminate the release of osteophilic (89)Zr(4+) and lead to a safer PET tracer with reduced background radiation dose. Herein, we present an octadentate chelator 3,4,3-(LI-1,2-HOPO) (or HOPO) as a potentially superior alternative to DFO. The HOPO ligand formed a 1:1 Zr-HOPO complex that was evaluated experimentally and theoretically. The stability of (89)Zr-HOPO matched or surpassed that of (89)Zr-DFO in every experiment. In healthy mice, (89)Zr-HOPO cleared the body rapidly with no signs of demetalation. Ultimately, HOPO has the potential to replace DFO as the chelator of choice for (89)Zr-based PET imaging agents.
Figures

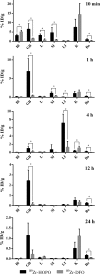

References
-
- Sinicropi M.; Amantea D.; Caruso A.; Saturnino C. Chemical and Biological Properties of Toxic Metals and Use of Chelating Agents for the Pharmacological Treatment of Metal Poisoning. Arch. Toxicol. 2010, 84, 501–520. - PubMed
-
- Baran E. J. Chelation Therapies: A Chemical and Biochemical Perspective. Curr. Med. Chem. 2010, 17, 3658–3672. - PubMed
-
- Price E. W.; Orvig C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

