Clostridium difficile spore biology: sporulation, germination, and spore structural proteins
- PMID: 24814671
- PMCID: PMC4098856
- DOI: 10.1016/j.tim.2014.04.003
Clostridium difficile spore biology: sporulation, germination, and spore structural proteins
Abstract
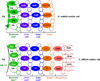
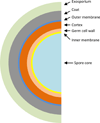
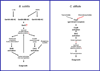
Clostridium difficile is a Gram-positive, spore-forming obligate anaerobe and a major nosocomial pathogen of worldwide concern. Owing to its strict anaerobic requirements, the infectious and transmissible morphotype is the dormant spore. In susceptible patients, C. difficile spores germinate in the colon to form the vegetative cells that initiate Clostridium difficile infections (CDI). During CDI, C. difficile induces a sporulation pathway that produces more spores; these spores are responsible for the persistence of C. difficile in patients and horizontal transmission between hospitalized patients. Although important to the C. difficile lifecycle, the C. difficile spore proteome is poorly conserved when compared to members of the Bacillus genus. Further, recent studies have revealed significant differences between C. difficile and Bacillus subtilis at the level of sporulation, germination, and spore coat and exosporium morphogenesis. In this review, the regulation of the sporulation and germination pathways and the morphogenesis of the spore coat and exosporium will be discussed.
Keywords: C. difficile spores; exosporium; germination; spore coat; sporulation.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Rupnik M, et al. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. - PubMed
-
- Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. - PubMed
-
- McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16:292–307. - PubMed
-
- Hernández-Rocha C, et al. Infecciones causadas por Clostridium difficile: una visión actualizada. Revista Chilena de Infectología. 2012;29:434–445. - PubMed
-
- Miller BA, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

