Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-κB decoy oligodeoxynucleotide: a preliminary report
- PMID: 24814879
- PMCID: PMC4081023
- DOI: 10.1016/j.actbio.2014.04.034
Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-κB decoy oligodeoxynucleotide: a preliminary report
Abstract
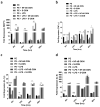
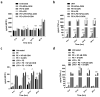
Total joint replacement (TJR) is very cost-effective surgery for end-stage arthritis. One important goal is to decrease the revision rate, mainly because TJR has been extended to younger patients. Continuous production of ultra-high molecular weight polyethylene (UHMWPE) wear particles induces macrophage infiltration and chronic inflammation, which can lead to periprosthetic osteolysis. Targeting individual pro-inflammatory cytokines directly has not reversed the osteolytic process in clinical trials, owing to compensatory up-regulation of other pro-inflammatory factors. It is hypothesized that targeting the important transcription factor NF-κB could mitigate the inflammatory response to wear particles, potentially diminishing osteolysis. In the current study, NF-κB activity in mouse RAW 264.7 and human THP1 macrophage cell lines, as well as primary mouse and human macrophages, was suppressed via competitive binding with double strand decoy oligodeoxynucleotide (ODN) containing an NF-κB binding element. It was found that macrophage exposure to UHMWPE particles induced multiple pro-inflammatory cytokine and chemokine expression, including TNF-α, MCP1, MIP1α and others. Importantly, the decoy ODN significantly suppressed the induced cytokine and chemokine expression in both murine and human macrophages, and resulted in suppression of macrophage recruitment. The strategic use of decoy NF-κB ODN, delivered locally, could potentially diminish particle-induced periprosthetic osteolysis.
Keywords: Macrophage; NF-κB decoy oligodeoxynucleotide; Periprosthetic osteolysis; Wear particles.
Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
NF-κB decoy oligodeoxynucleotide mitigates wear particle-associated bone loss in the murine continuous infusion model.Acta Biomater. 2016 Sep 1;41:273-81. doi: 10.1016/j.actbio.2016.05.038. Epub 2016 May 31. Acta Biomater. 2016. PMID: 27260104 Free PMC article.
-
Suppression of NF-κB signaling mitigates polyethylene wear particle-induced inflammatory response.Inflamm Cell Signal. 2014;1(4):e223. doi: 10.14800/ics.223. Inflamm Cell Signal. 2014. PMID: 26052541 Free PMC article.
-
Orthopaedic wear particle-induced bone loss and exogenous macrophage infiltration is mitigated by local infusion of NF-κB decoy oligodeoxynucleotide.J Biomed Mater Res A. 2017 Nov;105(11):3169-3175. doi: 10.1002/jbm.a.36169. Epub 2017 Sep 13. J Biomed Mater Res A. 2017. PMID: 28782280 Free PMC article.
-
Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target.Acta Biomater. 2014 Jan;10(1):1-10. doi: 10.1016/j.actbio.2013.09.034. Epub 2013 Oct 1. Acta Biomater. 2014. PMID: 24090989 Free PMC article. Review.
-
Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement.J R Soc Interface. 2014 Jan 29;11(93):20130962. doi: 10.1098/rsif.2013.0962. Print 2014 Apr 6. J R Soc Interface. 2014. PMID: 24478281 Free PMC article. Review.
Cited by
-
Deacylcynaropicrin Inhibits RANKL-Induced Osteoclastogenesis by Inhibiting NF-κB and MAPK and Promoting M2 Polarization of Macrophages.Front Pharmacol. 2019 Jun 7;10:599. doi: 10.3389/fphar.2019.00599. eCollection 2019. Front Pharmacol. 2019. PMID: 31231214 Free PMC article.
-
NFκB sensing IL-4 secreting mesenchymal stem cells mitigate the proinflammatory response of macrophages exposed to polyethylene wear particles.J Biomed Mater Res A. 2018 Oct;106(10):2744-2752. doi: 10.1002/jbm.a.36504. Epub 2018 Aug 7. J Biomed Mater Res A. 2018. PMID: 30084534 Free PMC article.
-
Protective effects of Asiatic acid against pelvic inflammatory disease in rats.Exp Ther Med. 2019 Jun;17(6):4687-4692. doi: 10.3892/etm.2019.7498. Epub 2019 Apr 17. Exp Ther Med. 2019. PMID: 31086602 Free PMC article.
-
Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis.Stem Cell Res Ther. 2017 Dec 6;8(1):277. doi: 10.1186/s13287-017-0730-z. Stem Cell Res Ther. 2017. PMID: 29212557 Free PMC article.
-
Involvement of NF-κB/NLRP3 axis in the progression of aseptic loosening of total joint arthroplasties: a review of molecular mechanisms.Naunyn Schmiedebergs Arch Pharmacol. 2022 Jul;395(7):757-767. doi: 10.1007/s00210-022-02232-4. Epub 2022 Apr 4. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35377011 Review.
References
-
- Tsao A, Jones L, Lewallen D Implant Wear Symposium Clinical Work G. What patient and surgical factors contribute to implant wear and osteolysis in total joint arthroplasty? The Journal of the American Academy of Orthopaedic Surgeons. 2008;16(Suppl 1):13. - PubMed
-
- Sochart D. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clinical orthopaedics and related research. 1999:135–50. - PubMed
-
- Johnston R. Current concepts: immunology. Monocytes and macrophages. The New England journal of medicine. 1988;318:747–52. - PubMed
-
- Schwarz E, Campbell D, Totterman S, Boyd A, O'Keefe R, Looney R. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2003;21:1049–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

