Unraveling the differences of the hydrolytic activity of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase: a quantum mechanics-molecular mechanics modeling study
- PMID: 24814976
- PMCID: PMC4051249
- DOI: 10.1021/jp412294r
Unraveling the differences of the hydrolytic activity of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase: a quantum mechanics-molecular mechanics modeling study
Abstract
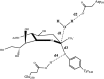
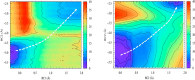
Chagas' disease, also known as American trypanosomiasis, is a lethal, chronic disease that currently affects more than 10 million people in Central and South America. The trans-sialidase from Trypanosoma cruzi (T. cruzi, TcTS) is a crucial enzyme for the survival of this parasite: sialic acids from the host are transferred to the cell surface glycoproteins of the trypanosome, thereby evading the host's immune system. On the other hand, the sialidase of T. rangeli (TrSA), which shares 70% sequence identity with TcTS, is a strict hydrolase and shows no trans-sialidase activity. Therefore, TcTS and TrSA represent an excellent framework to understand how different catalytic activities can be achieved with extremely similar structures. By means of combined quantum mechanics-molecular mechanics (QM/MM, SCC-DFTB/Amberff99SB) calculations and umbrella sampling simulations, we investigated the hydrolysis mechanisms of TcTS and TrSA and computed the free energy profiles of these reactions. The results, together with our previous computational investigations, are able to explain the catalytic mechanism of sialidases and describe how subtle differences in the active site make TrSA a strict hydrolase and TcTS a more efficient trans-sialidase.
Figures


Similar articles
-
Modulation of catalytic function by differential plasticity of the active site: case study of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase.Biochemistry. 2009 Apr 21;48(15):3398-406. doi: 10.1021/bi802230y. Biochemistry. 2009. PMID: 19216574 Free PMC article.
-
Free-energy computations identify the mutations required to confer trans-sialidase activity into Trypanosoma rangeli sialidase.Proteins. 2014 Mar;82(3):424-35. doi: 10.1002/prot.24408. Epub 2013 Oct 17. Proteins. 2014. PMID: 23999862
-
The high resolution structures of free and inhibitor-bound Trypanosoma rangeli sialidase and its comparison with T. cruzi trans-sialidase.J Mol Biol. 2003 Jan 24;325(4):773-84. doi: 10.1016/s0022-2836(02)01306-2. J Mol Biol. 2003. PMID: 12507479
-
Trypanosoma cruzi Trans-sialidase: structural features and biological implications.Subcell Biochem. 2014;74:181-201. doi: 10.1007/978-94-007-7305-9_8. Subcell Biochem. 2014. PMID: 24264246 Review.
-
Trypanosoma cruzi trans-sialidase as a drug target against Chagas disease (American trypanosomiasis).Future Med Chem. 2013 Oct;5(15):1889-900. doi: 10.4155/fmc.13.129. Future Med Chem. 2013. PMID: 24144418 Review.
Cited by
-
In silico structural characterization of protein targets for drug development against Trypanosoma cruzi.J Mol Model. 2016 Oct;22(10):244. doi: 10.1007/s00894-016-3115-9. Epub 2016 Sep 24. J Mol Model. 2016. PMID: 27665464
-
Comparative studies of catalytic pathways for Streptococcus pneumoniae sialidases NanA, NanB and NanC.Sci Rep. 2019 Feb 15;9(1):2157. doi: 10.1038/s41598-018-38131-z. Sci Rep. 2019. PMID: 30770840 Free PMC article.
References
-
- American Trypanosomiasis: Chagas Disease One Hundred Years of Research; Elsevier Insights, 1st ed.; Telleria J., Tibayrenc M., Eds.; Elsevier: London, 2010.
-
- Rassi A.; Rassi A.; Marin-Neto J. A. Chagas Disease. Lancet 2010, 375, 1388–1402. - PubMed
-
- Miller B. R.; Roitberg A. E. Trypanosoma Cruzi Trans-Sialidase as a Drug Target Against Chagas Disease (American Trypanosomiasis). Future Med. Chem. 2013, 5, 1889–1900. - PubMed
-
- Clayton J. Chagas Disease 101. Nature 2010, 465, S4–S5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

