Functional zonation of the rat adrenal cortex: the development and maintenance
- PMID: 24814991
- PMCID: PMC4104512
- DOI: 10.2183/pjab.90.163
Functional zonation of the rat adrenal cortex: the development and maintenance
Abstract
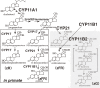
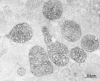
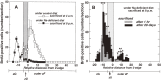
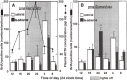
The adrenal cortex of mammals consists of three concentric zones, i.e., the zona glomerulosa (zG), the zona fasciculata (zF), and the zona reticularis (zR), which secrete mineralocorticoids, glucocorticoids, and adrenal androgens, respectively. In 1994, we identified immunohistochemically a new zone between zG and zF of the rat adrenal gland. The zone appeared to be devoid of any significant endocrine functions specific to adrenocortical zones, therefore, we designated the zone as "undifferentiated cell zone (zU)". Further, BrdU (5-bromo-2'-deoxyuridine)-incorporating cells (cells in S-phase) were concentrated at the outer region and the inner region of zU, and these cells proliferated and migrated bidirectionally: toward zG centrifugally and toward zF centripetally. We proposed that cells in and around zU are stem/progenitor cells of the rat adrenal cortex, maintaining functional zonation of the adrenal cortex. The view is consistent with observations reported recently that Sonic hedgehog (Shh), an important factor in embryonic development and adult stem cell maintenance, exists in zU of the rat adrenal gland and the Shh-containing cells seem to migrate bidirectionally.
Figures








References
-
- Eustachi, B. (1563) Opuscula Anatomica, Vincentius Luchinus, Venice.
-
- Addison, T. (1855) On the constitutional and local effect of disease of the supra-renal capsules. Samuel Highley, London.
-
- Orth, D.N., Kavacs, W.J. and Rowan, D.C. (1992) The adreal cortex. In Wilson’s Textbook of Endocrinology (eds. Wilson, J.D. and Foster, D.W.). Sanders, Philadelphia, pp. 489–619.
-
- Arnold J. (1866) Ein Beitrag zür feineren Struktur und dem Chemismus der Nebennieren. Arch. Pathol. Anat. Physiol. Klin. Med. 35, 64–107
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

