Systematic domain swaps of iterative, nonreducing polyketide synthases provide a mechanistic understanding and rationale for catalytic reprogramming
- PMID: 24815013
- PMCID: PMC4046768
- DOI: 10.1021/ja5007299
Systematic domain swaps of iterative, nonreducing polyketide synthases provide a mechanistic understanding and rationale for catalytic reprogramming
Abstract
Iterative, nonreducing polyketide synthases (NR-PKSs) are multidomain enzymes responsible for the construction of the core architecture of aromatic polyketide natural products in fungi. Engineering these enzymes for the production of non-native metabolites has been a long-standing goal. We conducted a systematic survey of in vitro "domain swapped" NR-PKSs using an enzyme deconstruction approach. The NR-PKSs were dissected into mono- to multidomain fragments and recombined as noncognate pairs in vitro, reconstituting enzymatic activity. The enzymes used in this study produce aromatic polyketides that are representative of the four main chemical features set by the individual NR-PKS: starter unit selection, chain-length control, cyclization register control, and product release mechanism. We found that boundary conditions limit successful chemistry, which are dependent on a set of underlying enzymatic mechanisms. Crucial for successful redirection of catalysis, the rate of productive chemistry must outpace the rate of spontaneous derailment and thioesterase-mediated editing. Additionally, all of the domains in a noncognate system must interact efficiently if chemical redirection is to proceed. These observations refine and further substantiate current understanding of the mechanisms governing NR-PKS catalysis.
Figures





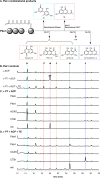




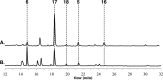

Similar articles
-
Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA.ACS Chem Biol. 2015 Jun 19;10(6):1443-9. doi: 10.1021/acschembio.5b00005. Epub 2015 Mar 10. ACS Chem Biol. 2015. PMID: 25714897 Free PMC article.
-
The Structural Enzymology of Iterative Aromatic Polyketide Synthases: A Critical Comparison with Fatty Acid Synthases.Annu Rev Biochem. 2018 Jun 20;87:503-531. doi: 10.1146/annurev-biochem-063011-164509. Annu Rev Biochem. 2018. PMID: 29925265 Review.
-
Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases.Int J Mol Sci. 2025 Aug 13;26(16):7801. doi: 10.3390/ijms26167801. Int J Mol Sci. 2025. PMID: 40869122 Free PMC article.
-
Structure and function of an iterative polyketide synthase thioesterase domain catalyzing Claisen cyclization in aflatoxin biosynthesis.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6246-51. doi: 10.1073/pnas.0913531107. Epub 2010 Mar 23. Proc Natl Acad Sci U S A. 2010. PMID: 20332208 Free PMC article.
-
Biosynthesis of aromatic polyketides in microorganisms using type II polyketide synthases.Microb Cell Fact. 2020 May 24;19(1):110. doi: 10.1186/s12934-020-01367-4. Microb Cell Fact. 2020. PMID: 32448179 Free PMC article. Review.
Cited by
-
The structural organization of substrate loading in iterative polyketide synthases.Nat Chem Biol. 2018 May;14(5):474-479. doi: 10.1038/s41589-018-0026-3. Epub 2018 Apr 2. Nat Chem Biol. 2018. PMID: 29610486 Free PMC article.
-
Synthetic biology of fungal natural products.Front Microbiol. 2015 Jul 30;6:775. doi: 10.3389/fmicb.2015.00775. eCollection 2015. Front Microbiol. 2015. PMID: 26284053 Free PMC article. Review.
-
Use of a biosynthetic intermediate to explore the chemical diversity of pseudo-natural fungal polyketides.Nat Chem. 2015 Sep;7(9):737-43. doi: 10.1038/nchem.2308. Epub 2015 Aug 3. Nat Chem. 2015. PMID: 26291946
-
Late-Stage Conversion of Diphenylphosphonate to Fluorophosphonate Probes for the Investigation of Serine Hydrolases.Cell Chem Biol. 2019 Jun 20;26(6):878-884.e8. doi: 10.1016/j.chembiol.2019.02.020. Epub 2019 Apr 11. Cell Chem Biol. 2019. PMID: 30982751 Free PMC article.
-
Pathway engineering in yeast for synthesizing the complex polyketide bikaverin.Nat Commun. 2020 Dec 3;11(1):6197. doi: 10.1038/s41467-020-19984-3. Nat Commun. 2020. PMID: 33273470 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

