Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light
- PMID: 24815311
- PMCID: PMC6270770
- DOI: 10.3390/molecules19055925
Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light
Abstract
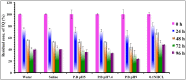
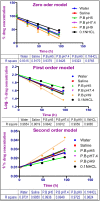
Thymoquinone (TQ) is a potent anticancer phytochemical with confirmed in vitro efficacy. Its clinical use has not yet established, and very few reports have documented its formulation. There also are no reports about the aqueous solubility and stability of this valuable drug, despite their direct correlation with the in vivo efficacy. In the current research, we have established and validated a stability-indicating HPLC method for the detection of TQ and its degradation products under different conditions. We then investigated the solubility and stability profiles of TQ in aqueous solutions. The stability study was aimed to determine the effect of pH, solvent type and light on the degradation process of TQ, along with the investigation of the kinetics of this degradation. The solubility of TQ varied in different aqueous solvents, and might be compromised due to stability issues. However, these findings confirm that the aqueous solubility is not the major obstacle for the drug formulations mainly due to the considerable water solubility (>500 μg/mL) that may be enough to exert pharmacologic effects if administered via parenteral route. Stability study results showed a very low stability profile of TQ in all the aqueous solutions with rapid degradation that varied with solvent type. The study of the degradation kinetics showed a significant effect of pH on the degradation process. The process followed first order kinetics at more acidic and alkaline pH values, and second order kinetics at pH 5-7.4, regardless of the solvent type. The results also expressed that light has a greater impact on the stability of TQ as a shorter period of exposure led to severe degradation, independent of the solution pH and solvent type. Our results also addressed some discrepancies in previously published researches regarding the formulation and quantification of TQ with suggested solutions. Overall, the current study concludes that TQ is unstable in aqueous solutions, particularly at an alkaline pH, in addition to presenting severe light sensitivity. This data indicates the inappropriateness of aqueous solutions as pharmaceutical vehicles for TQ preparations. To the best of our knowledge, this is the first study describing TQ aqueous solubility and stability that may lead to the development of a stable and effective TQ formulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Pre-formulation and chemical stability studies of penethamate, a benzylpenicillin ester prodrug, in aqueous vehicles.Drug Dev Ind Pharm. 2012 Jan;38(1):55-63. doi: 10.3109/03639045.2011.590497. Epub 2011 Jun 23. Drug Dev Ind Pharm. 2012. PMID: 21696334
-
Development of a lyophilised RH1 formulation: a novel DT diaphorase activated alkylating agent.J Pharm Pharmacol. 2002 Apr;54(4):487-92. doi: 10.1211/0022357021778754. J Pharm Pharmacol. 2002. PMID: 11999125
-
Solubility Determination, Hansen Solubility Parameters and Thermodynamic Evaluation of Thymoquinone in (Isopropanol + Water) Compositions.Molecules. 2021 May 26;26(11):3195. doi: 10.3390/molecules26113195. Molecules. 2021. PMID: 34073527 Free PMC article.
-
Thymoquinone-based nanotechnology for cancer therapy: promises and challenges.Drug Discov Today. 2018 May;23(5):1089-1098. doi: 10.1016/j.drudis.2018.01.043. Epub 2018 Jan 31. Drug Discov Today. 2018. PMID: 29374534 Review.
-
Nanocarriers: more than tour de force for thymoquinone.Expert Opin Drug Deliv. 2020 Apr;17(4):479-494. doi: 10.1080/17425247.2020.1730808. Epub 2020 Feb 20. Expert Opin Drug Deliv. 2020. PMID: 32077770 Review.
Cited by
-
Advances in research on the relationship between thymoquinone and pancreatic cancer.Front Oncol. 2023 Jan 4;12:1092020. doi: 10.3389/fonc.2022.1092020. eCollection 2022. Front Oncol. 2023. PMID: 36686732 Free PMC article. Review.
-
Activation of NQO-1 mediates the augmented contractions of isolated arteries due to biased activity of soluble guanylyl cyclase in their smooth muscle.Naunyn Schmiedebergs Arch Pharmacol. 2018 Nov;391(11):1221-1235. doi: 10.1007/s00210-018-1548-7. Epub 2018 Jul 30. Naunyn Schmiedebergs Arch Pharmacol. 2018. PMID: 30062552
-
Fabrication of Thymoquinone and Ascorbic Acid-Loaded Spanlastics Gel for Hyperpigmentation: In Vitro Release, Cytotoxicity, and Skin Permeation Studies.Pharmaceutics. 2025 Jan 2;17(1):48. doi: 10.3390/pharmaceutics17010048. Pharmaceutics. 2025. PMID: 39861696 Free PMC article.
-
Thymoquinone: A Promising Therapeutic Agent for the Treatment of Colorectal Cancer.Curr Issues Mol Biol. 2023 Dec 23;46(1):121-139. doi: 10.3390/cimb46010010. Curr Issues Mol Biol. 2023. PMID: 38248312 Free PMC article. Review.
-
Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer.Pharmaceutics. 2022 Jan 9;14(1):153. doi: 10.3390/pharmaceutics14010153. Pharmaceutics. 2022. PMID: 35057049 Free PMC article.
References
-
- Severina I.I., Severin F.F., Korshunova G.A., Sumbatyan N.V., Ilyasova T.M., Simonyan R.A., Rogov A.G., Trendeleva T.A., Zvyagilskaya R.A., Dugina V.B., et al. In search of novel highly active mitochondria-targeted antioxidants: Thymoquinone and its cationic derivatives. FEBS Lett. 2013;587:2018–2024. doi: 10.1016/j.febslet.2013.04.043. - DOI - PubMed
-
- Zubair H., Khan H.Y., Sohail A., Azim S., Ullah M.F., Ahmad A., Sarkar F.H., Hadi S.M. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: Putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:e660. doi: 10.1038/cddis.2013.172. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

