Endoplasmic reticulum stress mediates amyloid β neurotoxicity via mitochondrial cholesterol trafficking
- PMID: 24815354
- PMCID: PMC4076561
- DOI: 10.1016/j.ajpath.2014.03.014
Endoplasmic reticulum stress mediates amyloid β neurotoxicity via mitochondrial cholesterol trafficking
Abstract
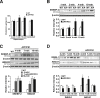
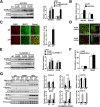
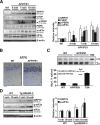
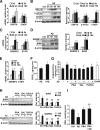
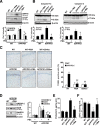
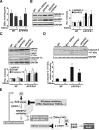
Disrupted cholesterol homeostasis has been reported in Alzheimer disease and is thought to contribute to disease progression by promoting amyloid β (Aβ) accumulation. In particular, mitochondrial cholesterol enrichment has been shown to sensitize to Aβ-induced neurotoxicity. However, the molecular mechanisms responsible for the increased cholesterol levels and its trafficking to mitochondria in Alzheimer disease remain poorly understood. Here, we show that endoplasmic reticulum (ER) stress triggered by Aβ promotes cholesterol synthesis and mitochondrial cholesterol influx, resulting in mitochondrial glutathione (mGSH) depletion in older age amyloid precursor protein/presenilin-1 (APP/PS1) mice. Mitochondrial cholesterol accumulation was associated with increased expression of mitochondrial-associated ER membrane proteins, which favor cholesterol translocation from ER to mitochondria along with specific cholesterol carriers, particularly the steroidogenic acute regulatory protein. In vivo treatment with the ER stress inhibitor 4-phenylbutyric acid prevented mitochondrial cholesterol loading and mGSH depletion, thereby protecting APP/PS1 mice against Aβ-induced neurotoxicity. Similar protection was observed with GSH ethyl ester administration, which replenishes mGSH without affecting the unfolded protein response, thus positioning mGSH depletion downstream of ER stress. Overall, these results indicate that Aβ-mediated ER stress and increased mitochondrial cholesterol trafficking contribute to the pathologic progression observed in old APP/PS1 mice, and that ER stress inhibitors may be explored as therapeutic agents for Alzheimer disease.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sparks D.L., Scheff S.W., Hunsaker J.C., 3rd, Liu H., Landers T., Gross D.R. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

