Cyclooxygenases 1 and 2 differentially regulate blood pressure and cerebrovascular responses to acute and chronic intermittent hypoxia: implications for sleep apnea
- PMID: 24815497
- PMCID: PMC4309085
- DOI: 10.1161/JAHA.114.000875
Cyclooxygenases 1 and 2 differentially regulate blood pressure and cerebrovascular responses to acute and chronic intermittent hypoxia: implications for sleep apnea
Abstract
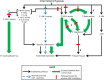
Background: Obstructive sleep apnea (OSA) is associated with increased risk of cardiovascular and cerebrovascular disease resulting from intermittent hypoxia (IH)-induced inflammation. Cyclooxygenase (COX)-formed prostanoids mediate the inflammatory response, and regulate blood pressure and cerebral blood flow (CBF), but their role in blood pressure and CBF responses to IH is unknown. Therefore, this study's objective was to determine the role of prostanoids in cardiovascular and cerebrovascular responses to IH.
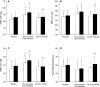
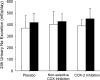
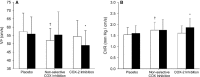
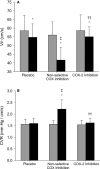
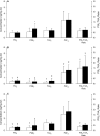
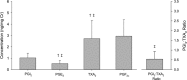
Methods and results: Twelve healthy, male participants underwent three, 6-hour IH exposures. For 4 days before each IH exposure, participants ingested a placebo, indomethacin (nonselective COX inhibitor), or Celebrex(®) (selective COX-2 inhibitor) in a double-blind, randomized, crossover study design. Pre- and post-IH blood pressure, CBF, and urinary prostanoids were assessed. Additionally, blood pressure and urinary prostanoids were assessed in newly diagnosed, untreated OSA patients (n=33). Nonselective COX inhibition increased pre-IH blood pressure (P ≤ 0.04) and decreased pre-IH CBF (P=0.04) while neither physiological variable was affected by COX-2 inhibition (P ≥ 0.90). Post-IH, MAP was elevated (P ≤ 0.05) and CBF was unchanged with placebo and nonselective COX inhibition. Selective COX-2 inhibition abrogated the IH-induced MAP increase (P=0.19), but resulted in lower post-IH CBF (P=0.01). Prostanoids were unaffected by IH, except prostaglandin E2 was elevated with the placebo (P=0.02). Finally, OSA patients had elevated blood pressure (P ≤ 0.4) and COX-1 formed thromboxane A2 concentrations (P=0.02).
Conclusions: COX-2 and COX-1 have divergent roles in modulating vascular responses to acute and chronic IH. Moreover, COX-1 inhibition may mitigate cardiovascular and cerebrovascular morbidity in OSA.
Clinical trial registration url: www.clinicaltrials.gov. Unique identifier: NCT01280006.
Keywords: blood pressure; cerebrovascular circulation; intermittent hypoxia; obstructive sleep apnea; prostaglandins.
Figures


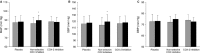
References
-
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an american Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In Collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008; 118:1080-1111. - PubMed
-
- Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011; 37:119-128. - PubMed
-
- Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin‐angiotensin‐system dependent mechanism. Hypertension. 2010; 56:369-377. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

