A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder
- PMID: 24815533
- PMCID: PMC4026396
- DOI: 10.1089/cap.2013.0096
A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder
Abstract
Objective: The purpose of this study was to evaluate the efficacy and safety of duloxetine fixed dose in the treatment of children (7-11 years) and adolescents (12-17 years) with major depressive disorder (MDD).
Methods: Patients (n=463) in this 36 week study (10 week acute and 26 week extension treatment) received duloxetine 60 mg QD (n=108), duloxetine 30 mg QD (n=116), fluoxetine 20 mg QD (n=117, active control), or placebo (n=122). Measures included: Children's Depression Rating Scale-Revised (CDRS-R), treatment-emergent adverse events (TEAEs), and Columbia-Suicide Severity Rating Scale (C-SSRS).
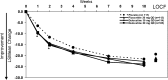
Results: Neither active drug (duloxetine or fluoxetine) separated significantly (p<0.05) from placebo on mean change from baseline to end-point (10 weeks) on the CDRS-R total score. Total TEAEs and discontinuation for AEs were significantly (p<0.05) higher only for the duloxetine 60 mg group versus the placebo group during acute treatment. No clinically significant electrocardiogram (ECG) or laboratory abnormalities were observed, and no completed suicides or deaths occurred during the study. A total of 7 (6.7%) duloxetine 60 mg, 6 (5.2%) duloxetine 30 mg, 9 (8.0%) fluoxetine, and 11 (9.4%) placebo patients had worsening of suicidal ideation from baseline during acute treatment. Of the patients with suicidal ideation at baseline, 13/16 (81%) duloxetine 60 mg, 16/17 (94%) duloxetine 30 mg, 11/16 (69%) fluoxetine, and 13/15 (87%) placebo had improvement in suicidal ideation at end-point during acute treatment. One fluoxetine, one placebo, and six duloxetine patients had treatment-emergent suicidal behavior during the 36 week study.
Conclusions: Trial results were inconclusive, as neither the investigational drug (duloxetine) nor the active control (fluoxetine) separated from placebo on the CDRS-R at 10 weeks. No new duloxetine safety signals were identified relative to those seen in adults. Clinical Trial Registry Number ( www.ClinicalTrials.gov ): NCT00849693.
Figures
References
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000
-
- Atkinson SD, Prakash A, Zhang Q, Pangallo BA, Bangs ME, Emslie GJ, March JS: A double-blind, efficacy and safety study of duloxetine flexible doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:180–189, 2014 - PubMed
-
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 - PubMed
-
- Cymbalta Full Prescribing Information. Available at http://pi.lilly.com/us/Cymbalta-pi.pdf Accessed March1, 2014
-
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 41:1205–1215, 2002 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

