Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data
- PMID: 24815805
- PMCID: PMC6637757
- DOI: 10.1016/S2213-2600(14)70041-4
Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data
Abstract
Background: Neuraminidase inhibitors were widely used during the 2009-10 influenza A H1N1 pandemic, but evidence for their effectiveness in reducing mortality is uncertain. We did a meta-analysis of individual participant data to investigate the association between use of neuraminidase inhibitors and mortality in patients admitted to hospital with pandemic influenza A H1N1pdm09 virus infection.
Methods: We assembled data for patients (all ages) admitted to hospital worldwide with laboratory confirmed or clinically diagnosed pandemic influenza A H1N1pdm09 virus infection. We identified potential data contributors from an earlier systematic review of reported studies addressing the same research question. In our systematic review, eligible studies were done between March 1, 2009 (Mexico), or April 1, 2009 (rest of the world), until the WHO declaration of the end of the pandemic (Aug 10, 2010); however, we continued to receive data up to March 14, 2011, from ongoing studies. We did a meta-analysis of individual participant data to assess the association between neuraminidase inhibitor treatment and mortality (primary outcome), adjusting for both treatment propensity and potential confounders, using generalised linear mixed modelling. We assessed the association with time to treatment using time-dependent Cox regression shared frailty modelling.
Findings: We included data for 29,234 patients from 78 studies of patients admitted to hospital between Jan 2, 2009, and March 14, 2011. Compared with no treatment, neuraminidase inhibitor treatment (irrespective of timing) was associated with a reduction in mortality risk (adjusted odds ratio [OR] 0·81; 95% CI 0·70-0·93; p=0·0024). Compared with later treatment, early treatment (within 2 days of symptom onset) was associated with a reduction in mortality risk (adjusted OR 0·48; 95% CI 0·41-0·56; p<0·0001). Early treatment versus no treatment was also associated with a reduction in mortality (adjusted OR 0·50; 95% CI 0·37-0·67; p<0·0001). These associations with reduced mortality risk were less pronounced and not significant in children. There was an increase in the mortality hazard rate with each day's delay in initiation of treatment up to day 5 as compared with treatment initiated within 2 days of symptom onset (adjusted hazard ratio [HR 1·23] [95% CI 1·18-1·28]; p<0·0001 for the increasing HR with each day's delay).
Interpretation: We advocate early instigation of neuraminidase inhibitor treatment in adults admitted to hospital with suspected or proven influenza infection.
Funding: F Hoffmann-La Roche.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
Between October, 2007, and September, 2010, JSN-V-T did ad-hoc paid consultancy and lecturing for several influenza vaccine manufacturers (Sanofi Pasteur MSD, Sanofi Pasteur, GlaxoSmithKline, Baxter AG, Solvay, Novartis) and manufacturers of neuraminidase inhibitors (F Hoffmann-La Roche: oseltamivir [Tamiflu] and GlaxoSmithKline: zanamivir [Relenza]). He is a former employee of SmithKline Beecham (now part of GlaxoSmithKline), Roche Products (UK), and Sanofi Pasteur MSD, all before 2005. He has no outstanding interests related to shares, share options, or accrued pension rights in any of these companies. He is in receipt of current or recent research funding, related to influenza vaccination from GlaxoSmithKline and AstraZeneca and non-financial support (travel) from Baxter AG. His brother became an employee of GlaxoSmithKline in January, 2014. JL-B is Statistical Editor of the Cochrane Skin Group. PRM is the recipient of the unrestricted educational grant for research in the area of pandemic influenza from F Hoffman-La Roche, used to fund this work. She has also received travel grants from F Hoffman-La Roche and its subsidiaries to attend clinical seminars to present this work. RB has received financial support from CSL, Sanofi, GlaxoSmithKline, Novartis, Roche, and Wyeth to do research and present at scientific meetings. Any funding received is directed to an NCIRS research account at The Children’s Hospital at Westmead, NSW, Australia, and is not personally accepted by RB. All other named authors declare that they have no competing interests.
Figures
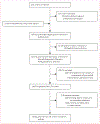
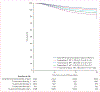
Comment in
-
Study claiming Tamiflu saved lives was based on "flawed" analysis.BMJ. 2014 Mar 18;348:g2228. doi: 10.1136/bmj.g2228. BMJ. 2014. PMID: 24646608 No abstract available.
-
Authors' reply to Mark Jones's second critique of the study by Muthuri and colleagues reported in The BMJ.BMJ. 2014 Apr 30;348:g3004. doi: 10.1136/bmj.g3004. BMJ. 2014. PMID: 24788389 No abstract available.
-
Authors' reply to Mark Jones's critique of the study by Muthuri and colleagues reported in The BMJ.BMJ. 2014 Apr 30;348:g2990. doi: 10.1136/bmj.g2990. BMJ. 2014. PMID: 24788485 No abstract available.
-
Mark Jones's reply to Myles and Leonardi-Bee's response to his critique of their paper reported in The BMJ.BMJ. 2014 Apr 30;348:g3001. doi: 10.1136/bmj.g3001. BMJ. 2014. PMID: 24788584 No abstract available.
-
Effectiveness of neuraminidase inhibitors for severe influenza.Lancet Respir Med. 2014 May;2(5):346-8. doi: 10.1016/S2213-2600(14)70068-2. Epub 2014 Mar 19. Lancet Respir Med. 2014. PMID: 24815800 No abstract available.
-
Statistical and methodological concerns about the beneficial effect of neuraminidase inhibitors on mortality.Lancet Respir Med. 2014 Jul;2(7):e10. doi: 10.1016/S2213-2600(14)70127-4. Epub 2014 Jun 16. Lancet Respir Med. 2014. PMID: 24948429 No abstract available.
-
Statistical and methodological concerns about the beneficial effect of neuraminidase inhibitors on mortality.Lancet Respir Med. 2014 Jul;2(7):e9-e10. doi: 10.1016/S2213-2600(14)70126-2. Epub 2014 Jun 16. Lancet Respir Med. 2014. PMID: 24948431 No abstract available.
-
Statistical and methodological concerns about the beneficial effect of neuraminidase inhibitors on mortality.Lancet Respir Med. 2014 Jul;2(7):e10-2. doi: 10.1016/S2213-2600(14)70137-7. Epub 2014 Jun 16. Lancet Respir Med. 2014. PMID: 24948433 No abstract available.
-
Statistical and methodological concerns about the beneficial effect of neuraminidase inhibitors on mortality.Lancet Respir Med. 2014 Jul;2(7):e8-9. doi: 10.1016/S2213-2600(14)70115-8. Epub 2014 Jun 16. Lancet Respir Med. 2014. PMID: 24948434 No abstract available.
References
-
- Donner B, Bader-Weder S, Schwarz R, Peng MM, Smith JR, Niranjan V. Safety profile of oseltamivir during the 2009 influenza pandemic. Pharmacoepidemiol Drug Saf 2011; 20: 532–43. - PubMed
-
- Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006; 85: 278–87 - PubMed
-
- Chemaly RF, Torres HA, Aguilera EA, et al. Neuraminidase inhibitors improve outcome of patients with leukemia and influenza: an observational study. Clin Infect Dis 2007; 44: 964–67 - PubMed
-
- Hassan K, McGeer A, Green KA, et al. Antiviral therapy improves outcomes of influenza infections in patients requiring admission to intensive care. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA; Sept 12–15, 2009. V-537
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

