Crystallographic and kinetic study of riboflavin synthase from Brucella abortus, a chemotherapeutic target with an enhanced intrinsic flexibility
- PMID: 24816110
- PMCID: PMC4014124
- DOI: 10.1107/S1399004714005161
Crystallographic and kinetic study of riboflavin synthase from Brucella abortus, a chemotherapeutic target with an enhanced intrinsic flexibility
Abstract
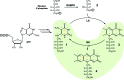
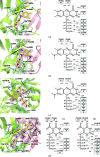
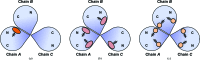
Riboflavin synthase (RS) catalyzes the last step of riboflavin biosynthesis in microorganisms and plants, which corresponds to the dismutation of two molecules of 6,7-dimethyl-8-ribityllumazine to yield one molecule of riboflavin and one molecule of 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione. Owing to the absence of this enzyme in animals and the fact that most pathogenic bacteria show a strict dependence on riboflavin biosynthesis, RS has been proposed as a potential target for antimicrobial drug development. Eubacterial, fungal and plant RSs assemble as homotrimers lacking C3 symmetry. Each monomer can bind two substrate molecules, yet there is only one active site for the whole enzyme, which is located at the interface between two neighbouring chains. This work reports the crystallographic structure of RS from the pathogenic bacterium Brucella abortus (the aetiological agent of the disease brucellosis) in its apo form, in complex with riboflavin and in complex with two different product analogues, being the first time that the structure of an intact RS trimer with bound ligands has been solved. These crystal models support the hypothesis of enhanced flexibility in the particle and also highlight the role of the ligands in assembling the unique active site. Kinetic and binding studies were also performed to complement these findings. The structural and biochemical information generated may be useful for the rational design of novel RS inhibitors with antimicrobial activity.
Keywords: 6,7-dimethyl-8-ribityllumazine; enzyme–ligand complex; inhibition by substrate and product; vitamin B2.
Figures


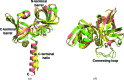




Similar articles
-
Evolution of vitamin B2 biosynthesis: 6,7-dimethyl-8-ribityllumazine synthases of Brucella.J Bacteriol. 2006 Sep;188(17):6135-42. doi: 10.1128/JB.00207-06. J Bacteriol. 2006. PMID: 16923880 Free PMC article.
-
Biosynthesis of vitamin B2: Structure and mechanism of riboflavin synthase.Arch Biochem Biophys. 2008 Jun 15;474(2):252-65. doi: 10.1016/j.abb.2008.02.008. Epub 2008 Feb 14. Arch Biochem Biophys. 2008. PMID: 18298940 Review.
-
Studies on the reaction mechanism of riboflavin synthase: X-ray crystal structure of a complex with 6-carboxyethyl-7-oxo-8-ribityllumazine.Structure. 2002 Oct;10(10):1371-81. doi: 10.1016/s0969-2126(02)00864-x. Structure. 2002. PMID: 12377123
-
The structure of the N-terminal domain of riboflavin synthase in complex with riboflavin at 2.6A resolution.J Mol Biol. 2003 Aug 29;331(5):1053-63. doi: 10.1016/s0022-2836(03)00844-1. J Mol Biol. 2003. PMID: 12927541
-
Biosynthesis of riboflavin.Vitam Horm. 2001;61:1-49. doi: 10.1016/s0083-6729(01)61001-x. Vitam Horm. 2001. PMID: 11153262 Review.
Cited by
-
Identification of the phosphatase essential for riboflavin biosynthesis in Aquifex aeolicus.J Biol Chem. 2025 May;301(5):108443. doi: 10.1016/j.jbc.2025.108443. Epub 2025 Mar 25. J Biol Chem. 2025. PMID: 40147773 Free PMC article.
-
Role of riboflavin biosynthesis gene duplication and transporter in Aeromonas salmonicida virulence in marine teleost fish.Virulence. 2023 Dec;14(1):2187025. doi: 10.1080/21505594.2023.2187025. Virulence. 2023. PMID: 36895132 Free PMC article.
-
Small Molecule Inhibitors against the Bacterial Pathogen Brucella.Curr Med Chem. 2024;31(27):4267-4285. doi: 10.2174/0929867331666230915153910. Curr Med Chem. 2024. PMID: 37718522 Review.
-
Inhibitors of riboflavin biosynthetic pathway enzymes as potential antibacterial drugs.Front Mol Biosci. 2023 Jul 11;10:1228763. doi: 10.3389/fmolb.2023.1228763. eCollection 2023. Front Mol Biosci. 2023. PMID: 37496776 Free PMC article. Review.
-
The Next Generation Scientist program: capacity-building for future scientific leaders in low- and middle-income countries.BMC Med Educ. 2018 Oct 10;18(1):233. doi: 10.1186/s12909-018-1331-y. BMC Med Educ. 2018. PMID: 30305069 Free PMC article.
References
-
- Akaike, H. (1974). IEEE Trans. Automat. Contr. 19, 716–723.
-
- Beach, R. & Plaut, G. W. (1969). Tetrahedron Lett., pp. 3489–3492. - PubMed
-
- Berguer, P. M., Mundiñano, J., Piazzon, I. & Goldbaum, F. A. (2006). J. Immunol. 176, 2366–2372. - PubMed
-
- Bricogne, G., Blanc, E., Brandl, M., Flensburg, C., Keller, P., Paciorek, W., Roversi, P., Sharff, A., Smart, O. S., Vonrhein, C. & Womack, T. O. (2011). BUSTER v.2.10.0. Cambridge: Global Phasing Ltd.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

