CXCR4 blockade induces atherosclerosis by affecting neutrophil function
- PMID: 24816217
- PMCID: PMC4418455
- DOI: 10.1016/j.yjmcc.2014.04.021
CXCR4 blockade induces atherosclerosis by affecting neutrophil function
Abstract
Aims: The SDF-1α/CXCR4 dyad was previously shown by us and others to be instrumental in intimal hyperplasia as well as early stage atherosclerosis. We here sought to investigate its impact on clinically relevant stages of atherosclerosis in mouse and man.
Methods and results: Immunohistochemical analysis of CXCR4 expression in human atherosclerotic lesions revealed a progressive accumulation of CXCR4(+) cells during plaque progression. To address causal involvement of CXCR4 in advanced stages of atherosclerosis we reconstituted LDLr(-/-) mice with autologous bone marrow infected with lentivirus encoding SDF-1α antagonist or CXCR4 degrakine, which effects proteasomal degradation of CXCR4. Functional CXCR4 blockade led to progressive plaque expansion with disease progression, while also promoting intraplaque haemorrhage. Moreover, CXCR4 knockdown was seen to augment endothelial adhesion of neutrophils. Concordant with this finding, inhibition of CXCR4 function increased adhesive capacity and reduced apoptosis of neutrophils and resulted in hyperactivation of circulating neutrophils. Compatible with a role of the neutrophil CXCR4 in end-stage atherosclerosis, CXCR4 expression by circulating neutrophils was lowered in patients with acute cardiovascular syndromes.
Conclusion: In conclusion, CXCR4 contributes to later stages of plaque progression by perturbing neutrophil function.
Keywords: CXCR4; SDF-1α; atherosclerosis; neutrophils; senescence.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures




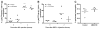

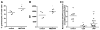
Similar articles
-
Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis.Circ Res. 2008 Feb 1;102(2):209-17. doi: 10.1161/CIRCRESAHA.107.160697. Epub 2007 Nov 8. Circ Res. 2008. PMID: 17991882
-
Mast cells mediate neutrophil recruitment during atherosclerotic plaque progression.Atherosclerosis. 2015 Aug;241(2):289-96. doi: 10.1016/j.atherosclerosis.2015.05.028. Epub 2015 Jun 3. Atherosclerosis. 2015. PMID: 26062988
-
Mitochondrial Oxidative Stress Promotes Atherosclerosis and Neutrophil Extracellular Traps in Aged Mice.Arterioscler Thromb Vasc Biol. 2017 Aug;37(8):e99-e107. doi: 10.1161/ATVBAHA.117.309580. Epub 2017 Jun 8. Arterioscler Thromb Vasc Biol. 2017. PMID: 28596373 Free PMC article.
-
Beyond Lipoprotein Receptors: Learning from Receptor Knockouts Mouse Models about New Targets for Reduction of the Atherosclerotic Plaque.Curr Mol Med. 2015;15(10):905-31. doi: 10.2174/1566524016666151123110310. Curr Mol Med. 2015. PMID: 26592248 Review.
-
Role of RNA-binding Proteins in Regulating Cell Adhesion and Progression of the Atherosclerotic Plaque and Plaque Erosion.Curr Atheroscler Rep. 2024 Nov 22;27(1):8. doi: 10.1007/s11883-024-01250-2. Curr Atheroscler Rep. 2024. PMID: 39576410 Review.
Cited by
-
Vascular CXCR4 Limits Atherosclerosis by Maintaining Arterial Integrity: Evidence From Mouse and Human Studies.Circulation. 2017 Jul 25;136(4):388-403. doi: 10.1161/CIRCULATIONAHA.117.027646. Epub 2017 Apr 27. Circulation. 2017. PMID: 28450349 Free PMC article.
-
Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR-/- Mice on Western Type Diet.PLoS One. 2015 Jun 19;10(6):e0130484. doi: 10.1371/journal.pone.0130484. eCollection 2015. PLoS One. 2015. PMID: 26090792 Free PMC article.
-
Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways.Antioxidants (Basel). 2022 Dec 29;12(1):72. doi: 10.3390/antiox12010072. Antioxidants (Basel). 2022. PMID: 36670934 Free PMC article.
-
Characteristics of peripheral blood cells are independently related to major adverse cardiovascular events after carotid endarterectomy.Atheroscler Plus. 2023 Jun 1;52:32-40. doi: 10.1016/j.athplu.2023.05.003. eCollection 2023 Jun. Atheroscler Plus. 2023. PMID: 37389152 Free PMC article.
-
Nicotinamide phosphoribosyltransferase aggravates inflammation and promotes atherosclerosis in ApoE knockout mice.Acta Pharmacol Sin. 2019 Sep;40(9):1184-1192. doi: 10.1038/s41401-018-0207-3. Epub 2019 Mar 4. Acta Pharmacol Sin. 2019. PMID: 30833708 Free PMC article.
References
-
- Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. - PubMed
-
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22. - PubMed
-
- Shah PK. Molecular mechanisms of plaque instability. Curr Opin Lipidol. 2007;18:492–9. - PubMed
-
- Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–21. - PubMed
-
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

