The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma
- PMID: 24816638
- PMCID: PMC4016361
- DOI: 10.1371/journal.pone.0097414
The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma
Abstract
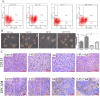
Hepatocellular carcinoma (HCC) is one of the few cancers in which a continuous increase in incidence has been observed over several years. Drug resistance is a major problem in the treatment of HCC. In the present study, we used salinomycin (Sal) and 5-fluorouracil (5-FU) combination therapy on HCC cell lines Huh7, LM3 and SMMC-7721 and nude mice subcutaneously tumor model to study whether Sal could increase the sensitivity of hepatoma cells to the traditional chemotherapeutic agent such as 5-FU. The combination of Sal and 5-FU resulted in a synergistic antitumor effect against liver tumors both in vitro and in vivo. Sal reversed the 5-FU-induced increase in CD133(+) EPCAM(+) cells, epithelial-mesenchymal transition and activation of the Wnt/β-catenin signaling pathway. The combination of Sal and 5-FU may provide us with a new approach to reverse drug resistant for the treatment of patients with HCC.
Conflict of interest statement
Figures
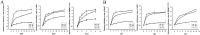



References
-
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1): 11–30. - PubMed
-
- Ikeda M, Okusaka T, Ueno H, Morizane C, Kojima Y, et al. (2008) Predictive factors of outcome and tumor response to systemic chemotherapy in patients with metastatic hepatocellular carcinoma. Jpn J Clin Oncol 38(10): 675–82. - PubMed
-
- Macdonald JS, Astrow AB (2001) Adjuvant therapy of colon cancer. Semin Oncol 28(1): 30–40. - PubMed
-
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, et al. (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352(22): 2302–13. - PubMed
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, et al. (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345(10): 725–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

