Novel approach to activity evaluation for release-active forms of anti-interferon-gamma antibodies based on enzyme-linked immunoassay
- PMID: 24816648
- PMCID: PMC4016219
- DOI: 10.1371/journal.pone.0097017
Novel approach to activity evaluation for release-active forms of anti-interferon-gamma antibodies based on enzyme-linked immunoassay
Retraction in
-
Retraction: Novel Approach to Activity Evaluation for Release-Active Forms of Anti-Interferon-Gamma Antibodies Based on Enzyme-Linked Immunoassay.PLoS One. 2018 May 3;13(5):e0197086. doi: 10.1371/journal.pone.0197086. eCollection 2018. PLoS One. 2018. PMID: 29723299 Free PMC article. No abstract available.
Abstract
Selection of a suitable assay to measure the activity of drug agents based on release-active forms of anti-interferon-gamma antibodies (RA forms of Abs) is an important step forward in the investigation of such agents. In this study, the enzyme-linked immunosorbent assay was utilized to examine the effect of RA forms of Abs specific for human interferon gamma on the interaction between monoclonal anti-interferon gamma antibodies and recombinant human interferon gamma. The experimental data and the results obtained by using relevant mathematical analysis showed that such RA forms of Abs are able to modulate the monoclonal antibody interaction with both soluble and immobilized (to the assay plate well) interferon gamma. These data demonstrated the importance of using relatively low concentrations of both soluble and plate-immobilized interferon gamma to detect the effects of RA forms of Abs to interferon gamma on the binding of monoclonal antibodies to interferon gamma. It has been suggested that the observed influence of RA forms of Abs on 'antibody-antigen' interaction could be used to detect and analyze the activity of drugs containing RA forms of Abs.
Conflict of interest statement
Figures
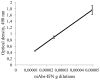

 from 1/li calculated from “A” curves and the equations for these linear functions where the slopes are equal to the dissociation constants for antigen-antibody reaction.
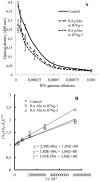
from 1/li calculated from “A” curves and the equations for these linear functions where the slopes are equal to the dissociation constants for antigen-antibody reaction.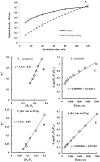
Similar articles
-
Use of Piezoelectric Immunosensors for Detection of Interferon-Gamma Interaction with Specific Antibodies in the Presence of Released-Active Forms of Antibodies to Interferon-Gamma.Sensors (Basel). 2016 Jan 20;16(1):96. doi: 10.3390/s16010096. Sensors (Basel). 2016. PMID: 26791304 Free PMC article.
-
Advanced approach to activity evaluation for released-active forms of antibodies to interferon-gamma by enzyme-linked immunoassay.J Immunoassay Immunochem. 2019;40(3):250-268. doi: 10.1080/15321819.2019.1567536. Epub 2019 Jan 20. J Immunoassay Immunochem. 2019. PMID: 30663507
-
Study of antigenic epitopes recognized by monoclonal antibodies to recombinant interferon-gamma.Biochem Biophys Res Commun. 1985 Apr 16;128(1):171-8. doi: 10.1016/0006-291x(85)91660-2. Biochem Biophys Res Commun. 1985. PMID: 2580528
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Anti-gamma interferon (IFN-gamma) monoclonal antibodies.Immunol Ser. 1993;59:319-28. Immunol Ser. 1993. PMID: 8461396 Review. No abstract available.
Cited by
-
Retraction: Novel Approach to Activity Evaluation for Release-Active Forms of Anti-Interferon-Gamma Antibodies Based on Enzyme-Linked Immunoassay.PLoS One. 2018 May 3;13(5):e0197086. doi: 10.1371/journal.pone.0197086. eCollection 2018. PLoS One. 2018. PMID: 29723299 Free PMC article. No abstract available.
-
Use of Piezoelectric Immunosensors for Detection of Interferon-Gamma Interaction with Specific Antibodies in the Presence of Released-Active Forms of Antibodies to Interferon-Gamma.Sensors (Basel). 2016 Jan 20;16(1):96. doi: 10.3390/s16010096. Sensors (Basel). 2016. PMID: 26791304 Free PMC article.
References
-
- Buss NAPS, Henderson SJ, McFarlane M, Shenton JM, de Haan L (2012) Monoclonal antibody therapeutics: history and future. Curr. Opin. Pharmacol, 12(5), 615. doi: 10.1016/j.coph.2012.08.001. - PubMed
-
- Frenzel A, Fröde D, Meyer T, Schirrmann T, Hust M (2012) Generating Recombinant Antibodies for Research, Diagnostics and Therapy Using Phage Display. Curr. Biotechnol. 1, 33–41. doi:10.2174/22111550111201010033
-
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F (2005) Monoclonal Versus Polyclonal Antibodies: Distinguishing Characteristics, Applications, and Information Resources. ILAR J. 46(3), 258–268. doi:10.1093/ilar.46.3.258 - PubMed
-
- Cao M, Cao P, Yan H, Lu W, Ren F, et al.. (2009) Construction, purification, and characterization of anti-BAFF scFv-Fc fusion antibody expressed in CHO/dhfr- cells. Appl Biochem Biotechnol. 157(3): , 562–74. doi: 10.1007/s12010-008-8434-6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

