Population-level impact of shorter-course regimens for tuberculosis: a model-based analysis
- PMID: 24816692
- PMCID: PMC4015982
- DOI: 10.1371/journal.pone.0096389
Population-level impact of shorter-course regimens for tuberculosis: a model-based analysis
Abstract
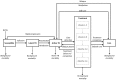
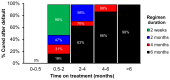
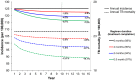
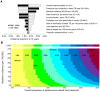
Despite current control efforts, global tuberculosis (TB) incidence is decreasing slowly. New regimens that can shorten treatment hold promise for improving treatment completion and success, but their impact on population-level transmission remains unclear. Earlier models projected that a four-month regimen could reduce TB incidence by 10% but assumed that an entire course of therapy must be completed to derive any benefit. We constructed a dynamic transmission model of TB disease calibrated to global estimates of incidence, prevalence, mortality, and treatment success. To account for the efficacy of partial treatment, we used data from clinical trials of early short-course regimens to estimate relapse rates among TB patients who completed one-third, one-half, two-thirds, and all of their first-line treatment regimens. We projected population-level incidence and mortality over 10 years, comparing standard six-month therapy to hypothetical shorter-course regimens with equivalent treatment success but fewer defaults. The impact of hypothetical four-month regimens on TB incidence after 10 years was smaller than estimated in previous modeling analyses (1.9% [95% uncertainty range 0.6-3.1%] vs. 10%). Impact on TB mortality was larger (3.5% at 10 years) but still modest. Transmission impact was most sensitive to the proportion of patients completing therapy: four-month therapy led to greater incidence reductions in settings where 25% of patients leave care ("default") over six months. Our findings remained robust under one-way variation of model parameters. These findings suggest that novel regimens that shorten treatment duration may have only a modest effect on TB transmission except in settings of very low treatment completion.
Conflict of interest statement
Figures
Similar articles
-
Priority-Setting for Novel Drug Regimens to Treat Tuberculosis: An Epidemiologic Model.PLoS Med. 2017 Jan 3;14(1):e1002202. doi: 10.1371/journal.pmed.1002202. eCollection 2017 Jan. PLoS Med. 2017. PMID: 28045934 Free PMC article.
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
-
Prospects for advancing tuberculosis control efforts through novel therapies.PLoS Med. 2006 Aug;3(8):e273. doi: 10.1371/journal.pmed.0030273. PLoS Med. 2006. PMID: 16866578 Free PMC article.
-
Shorter antibiotic regimens impact the control efforts in high tuberculosis burden regions of Taiwan.Int J Infect Dis. 2020 Aug;97:135-142. doi: 10.1016/j.ijid.2020.05.082. Epub 2020 May 29. Int J Infect Dis. 2020. PMID: 32474203
-
Shortened Treatment Regimens for Drug Sensitive TB.Indian J Pediatr. 2024 Jul;91(7):724-729. doi: 10.1007/s12098-023-04943-9. Epub 2023 Dec 15. Indian J Pediatr. 2024. PMID: 38100071 Review.
Cited by
-
Reasons for optimism in the search for new vaccines for tuberculosis.Epidemiol Infect. 2017 Jul;145(9):1750-1756. doi: 10.1017/S095026881700067X. Epub 2017 Apr 17. Epidemiol Infect. 2017. PMID: 28414012 Free PMC article.
-
Cost and cost-effectiveness of tuberculosis treatment shortening: a model-based analysis.BMC Infect Dis. 2016 Dec 1;16(1):726. doi: 10.1186/s12879-016-2064-3. BMC Infect Dis. 2016. PMID: 27905897 Free PMC article.
-
Mathematical Modelling and Tuberculosis: Advances in Diagnostics and Novel Therapies.Adv Med. 2015;2015:907267. doi: 10.1155/2015/907267. Epub 2015 Mar 15. Adv Med. 2015. PMID: 26556559 Free PMC article. Review.
-
The Impact and Cost-Effectiveness of a Four-Month Regimen for First-Line Treatment of Active Tuberculosis in South Africa.PLoS One. 2015 Dec 30;10(12):e0145796. doi: 10.1371/journal.pone.0145796. eCollection 2015. PLoS One. 2015. PMID: 26717007 Free PMC article.
-
Estimating the health and macroeconomic burdens of tuberculosis in India, 2021-2040: A fully integrated modelling study.PLoS Med. 2024 Dec 12;21(12):e1004491. doi: 10.1371/journal.pmed.1004491. eCollection 2024 Dec. PLoS Med. 2024. PMID: 39666614 Free PMC article.
References
-
- World Health Organization (2012) Global tuberculosis control 2012. Geneva: WHO.
-
- Flexner C (2007) HIV drug development: The next 25 years. Nat Rev Drug Discov 6: 959–966. - PubMed
-
- Koul A, Arnoult E, Lounis N, Guillemont J, Andries K (2011) The challenge of new drug discovery for tuberculosis. Nature 469: 483–490. - PubMed
-
- Spigelman M, Gillespie S (2006) Tuberculosis drug development pipeline: Progress and hope. Lancet 367: 945–947. - PubMed
-
- Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X (2010) Global tuberculosis drug development pipeline: The need and the reality. Lancet 375: 2100–2109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

