Role of HIV in amyloid metabolism
- PMID: 24816714
- PMCID: PMC4157652
- DOI: 10.1007/s11481-014-9546-0
Role of HIV in amyloid metabolism
Abstract
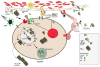
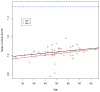
HIV infection has changed from an acute devastating disease to a more chronic illness due to combination anti-retroviral treatment (cART). In the cART era, the life expectancy of HIV-infected (HIV+) individuals has increased. More HIV + individuals are aging with current projections suggesting that 50% of HIV + individuals will be over 50 years old by 2015. With advancing age, HIV + individuals may be at increased risk of developing other potential neurodegenerative disorders [especially Alzheimer's disease (AD)]. Pathology studies have shown that HIV increases intra and possibly extracellular amyloid beta (Aβ42), a hallmark of AD. We review the synthesis and clearance of Aβ42; the effects of HIV on the amyloid pathway; and contrast the impact of AD and HIV on Aβ42 metabolism. Biomarker studies (cerebrospinal fluid AB and amyloid imaging) in HIV + participants have shown mixed results. CSF Aβ42 has been shown to be either normal or diminished in with HIV associated neurocognitive disorders (HAND). Amyloid imaging using [(11)C] PiB has also not demonstrated increased extracellular amyloid fibrillar deposits in HAND. We further demonstrate that Aβ42 deposition is not increased in older HIV + participants using [(11)C] PiB amyloid imaging. Together, these results suggest that HIV and aging each independently affect Aβ42 deposition with no significant interaction present. Older HIV + individuals are probably not at increased risk for developing AD. However, future longitudinal studies of older HIV + individuals using multiple modalities (including the combination of CSF markers and amyloid imaging) are necessary for investigating the effects of HIV on Aβ42 metabolism.
Conflict of interest statement
Figures

Similar articles
-
11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder.Arch Neurol. 2012 Jan;69(1):72-7. doi: 10.1001/archneurol.2011.761. Arch Neurol. 2012. PMID: 22232345 Free PMC article.
-
Reciprocal Predictive Relationships between Amyloid and Tau Biomarkers in Alzheimer's Disease Progression: An Empirical Model.J Neurosci. 2019 Sep 11;39(37):7428-7437. doi: 10.1523/JNEUROSCI.1056-19.2019. Epub 2019 Jul 26. J Neurosci. 2019. PMID: 31350262 Free PMC article.
-
Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort.J Alzheimers Dis. 2014;41(3):801-7. doi: 10.3233/JAD-132561. J Alzheimers Dis. 2014. PMID: 24705549
-
Fluid and PET biomarkers for amyloid pathology in Alzheimer's disease.Mol Cell Neurosci. 2019 Jun;97:3-17. doi: 10.1016/j.mcn.2018.12.004. Epub 2018 Dec 8. Mol Cell Neurosci. 2019. PMID: 30537535 Review.
-
Cerebrospinal fluid Abeta40 and Abeta42: natural course and clinical usefulness.Front Biosci. 2002 Apr 1;7:d997-1006. doi: 10.2741/A826. Front Biosci. 2002. PMID: 11897565 Review.
Cited by
-
HIV-1 promotes ubiquitination of the amyloidogenic C-terminal fragment of APP to support viral replication.Nat Commun. 2023 Jul 15;14(1):4227. doi: 10.1038/s41467-023-40000-x. Nat Commun. 2023. PMID: 37454116 Free PMC article.
-
Alzheimer's-Like Pathology at the Crossroads of HIV-Associated Neurological Disorders.Vaccines (Basel). 2021 Aug 21;9(8):930. doi: 10.3390/vaccines9080930. Vaccines (Basel). 2021. PMID: 34452054 Free PMC article. Review.
-
Fate of microglia during HIV-1 infection: From activation to senescence?Glia. 2017 Mar;65(3):431-446. doi: 10.1002/glia.23081. Epub 2016 Nov 26. Glia. 2017. PMID: 27888531 Free PMC article. Review.
-
Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection.PLoS One. 2014 Dec 26;9(12):e116081. doi: 10.1371/journal.pone.0116081. eCollection 2014. PLoS One. 2014. PMID: 25541953 Free PMC article.
-
Age-related neuroendocrine, cognitive, and behavioral co-morbidities are promoted by HIV-1 Tat expression in male mice.Aging (Albany NY). 2022 Jul 12;14(13):5345-5365. doi: 10.18632/aging.204166. Epub 2022 Jul 12. Aging (Albany NY). 2022. PMID: 35830469 Free PMC article.
References
-
- An SF, Giometto B, Groves M, et al. Axonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDS. J Neuropathol Exp Neurol. 1997;56:1262–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

