Novel polyglutamine model uncouples proteotoxicity from aging
- PMID: 24817148
- PMCID: PMC4016013
- DOI: 10.1371/journal.pone.0096835
Novel polyglutamine model uncouples proteotoxicity from aging
Abstract
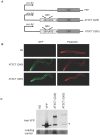
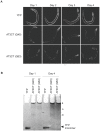
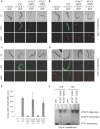
Polyglutamine expansions in certain proteins are the genetic determinants for nine distinct progressive neurodegenerative disorders and resultant age-related dementia. In these cases, neurodegeneration is due to the aggregation propensity and resultant toxic properties of the polyglutamine-containing proteins. We are interested in elucidating the underlying mechanisms of toxicity of the protein ataxin-3, in which a polyglutamine expansion is the genetic determinant for Machado-Joseph Disease (MJD), also referred to as spinocerebellar ataxia 3 (SCA3). To this end, we have developed a novel model for ataxin-3 protein aggregation, by expressing a disease-related polyglutamine-containing fragment of ataxin-3 in the genetically tractable body wall muscle cells of the model system C. elegans. Here, we demonstrate that this ataxin-3 fragment aggregates in a polyQ length-dependent manner in C. elegans muscle cells and that this aggregation is associated with cellular dysfunction. However, surprisingly, this aggregation and resultant toxicity was not influenced by aging. This is in contrast to polyglutamine peptides alone whose aggregation/toxicity is highly dependent on age. Thus, the data presented here not only describe a new polyglutamine model, but also suggest that protein context likely influences the cellular interactions of the polyglutamine-containing protein and thereby modulates its toxic properties.
Conflict of interest statement
Figures


References
-
- Masino L, Musi V, Menon RP, Fusi P, Kelly G, et al. (2003) Domain architecture of the polyglutamine protein ataxin-3: a globular domain followed by a flexible tail. FEBS Lett 549: 21–25. - PubMed
-
- Ellisdon AM, Thomas B, Bottomley SP (2006) The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J Biol Chem 281: 16888–16896. - PubMed
-
- Chow MK, Ellisdon AM, Cabrita LD, Bottomley SP (2004) Polyglutamine expansion in ataxin-3 does not affect protein stability: implications for misfolding and disease. J Biol Chem 279: 47643–47651. - PubMed
-
- Paulson HL, Perez MK, Trottier Y, Trojanowski JQ, Subramony SH, et al. (1997) Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 19: 333–344. - PubMed
-
- McLeod CJ, O'Keefe LV, Richards RI (2005) The pathogenic agent in Drosophila models of ‘polyglutamine’ diseases. Hum Mol Genet 14: 1041–1048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

