Inhaled hyperosmolar agents for bronchiectasis
- PMID: 24817558
- PMCID: PMC10804371
- DOI: 10.1002/14651858.CD002996.pub3
Inhaled hyperosmolar agents for bronchiectasis
Abstract
Background: Mucus retention in the lungs is a prominent feature of bronchiectasis. The stagnant mucus becomes chronically colonised with bacteria, which elicit a host neutrophilic response. This fails to eliminate the bacteria, and the large concentration of host-derived protease may contribute to the airway damage. The sensation of retained mucus is itself a cause of suffering, and the failure to maintain airway sterility probably contributes to the frequent respiratory infections experienced by many patients.Hypertonic saline inhalation is known to accelerate tracheobronchial clearance in many conditions, probably by inducing a liquid flux into the airway surface, which alters mucus rheology in a way favourable to mucociliary clearance. Inhaled dry powder mannitol has a similar effect. Such agents are an attractive approach to the problem of mucostasis, and deserve further clinical evaluation.
Objectives: To determine whether inhaled hyperosmolar substances are effective in the treatment of bronchiectasis.
Search methods: We searched the Cochrane Airways Group Specialised Register, trials registries, and the reference lists of included studies and review articles. Searches are current up to April 2014.
Selection criteria: Any randomised controlled trial (RCT) using hyperosmolar inhalation in patients with bronchiectasis not caused by cystic fibrosis.
Data collection and analysis: Two review authors assessed studies for suitability. We used standard methods recommended by The Cochrane Collaboration.
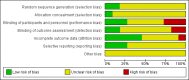
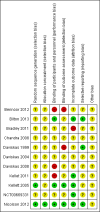
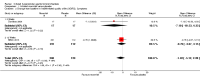
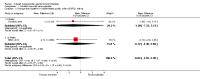
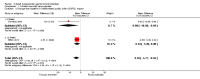
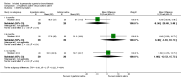
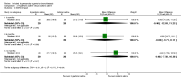
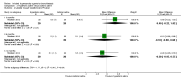
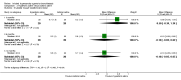
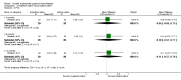
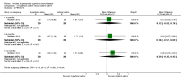
Main results: Eleven studies met the inclusion criteria of the review (1021 participants).Five studies on 833 participants compared inhaled mannitol with placebo but poor outcome reporting meant we could pool very little data and most outcomes were reported by only one study. One 12-month trial on 461 participants provided results for exacerbations and demonstrated an advantage for mannitol in terms of time to first exacerbation (median time to exacerbation 165 versus 124 days for mannitol and placebo respectively (hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.63 to 0.96, P = 0.022) and number of days on antibiotics for bronchiectasis exacerbations was significantly better with mannitol (risk ratio (RR) 0.76, 95%CI 0.58 to 1.00, P = 0.0496). However, exacerbation rate per year was not significantly different between mannitol and placebo (RR 0.92 95% CI 0.78 to 1.08). The quality of this evidence was rated as moderate. There was also an indication, from only three trials, again based on moderate quality evidence, that mannitol improves health-related quality of life (mean difference (MD) -2.05; 95% CI -3.69 to -0.40). An analysis of adverse events data, also based on moderate quality evidence, revealed no difference between mannitol and placebo (OR 0.96; 95% CI 0.61 to 1.51). Two additional small trials on 25 participants compared mannitol versus no treatment and the data from these studies were inconclusive.Four studies (combined N = 113) compared hypertonic saline versus isotonic saline. On most outcomes there were conflicting results and the opportunities for the statistical aggregation of data from studies was very limited. It is not possible to draw robust conclusions for this comparison and judgments should be reserved until further data are available.
Authors' conclusions: There is an indication from a single, large, unpublished study that inhaled mannitol increases time to first exacerbation in patients with bronchiectasis. In patients with near normal lung function, spirometry does not change dramatically with mannitol and adverse events are not more frequent than placebo. Further investigation is required in a patient population with impaired lung function.It is not possible to draw firm conclusions regarding the effect of nebulised hypertonic saline due to significant differences in the methodology, patient groups, and findings amongst the limited data available. The data suggest that it is unlikely to have benefit over isotonic saline in patients with milder disease, and hence future studies should test its use in those with more severe disease.
Conflict of interest statement
None known.
Figures

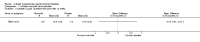
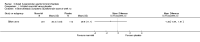
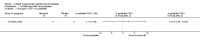
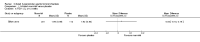

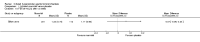
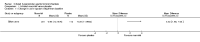

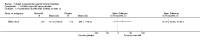





Update of
-
Inhaled hyperosmolar agents for bronchiectasis.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD002996. doi: 10.1002/14651858.CD002996.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 May 12;(5):CD002996. doi: 10.1002/14651858.CD002996.pub3. PMID: 16625566 Updated.
Similar articles
-
Inhaled hyperosmolar agents for bronchiectasis.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD002996. doi: 10.1002/14651858.CD002996.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 May 12;(5):CD002996. doi: 10.1002/14651858.CD002996.pub3. PMID: 16625566 Updated.
-
Inhaled hyperosmolar agents for bronchiectasis.Cochrane Database Syst Rev. 2002;(1):CD002996. doi: 10.1002/14651858.CD002996. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2006 Apr 19;(2):CD002996. doi: 10.1002/14651858.CD002996.pub2. PMID: 11869647 Updated.
-
Inhaled hyperosmolar agents for bronchiectasis.Cochrane Database Syst Rev. 2001;(2):CD002996. doi: 10.1002/14651858.CD002996. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2002;(1):CD002996. doi: 10.1002/14651858.CD002996. PMID: 11406058 Updated.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2015 Oct 9;(10):CD008649. doi: 10.1002/14651858.CD008649.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Feb 09;2:CD008649. doi: 10.1002/14651858.CD008649.pub3. PMID: 26451533 Updated.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
Cited by
-
Bronchiectasis in Primary Antibody Deficiencies: A Multidisciplinary Approach.Front Immunol. 2020 Mar 31;11:522. doi: 10.3389/fimmu.2020.00522. eCollection 2020. Front Immunol. 2020. PMID: 32296433 Free PMC article. Review.
-
Mycobacterium abscessus disease in lung transplant recipients: Diagnosis and management.J Clin Tuberc Other Mycobact Dis. 2017 Dec;9:10-18. doi: 10.1016/j.jctube.2017.08.002. J Clin Tuberc Other Mycobact Dis. 2017. PMID: 29276785 Free PMC article.
-
A 2 × 2 factorial, randomised, open-label trial to determine the clinical and cost-effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in adults with bronchiectasis: a protocol for the CLEAR clinical trial.Trials. 2019 Dec 19;20(1):747. doi: 10.1186/s13063-019-3766-9. Trials. 2019. PMID: 31856887 Free PMC article.
-
Challenges of diagnosing and managing bronchiectasis in resource-limited settings: a case study.Pan Afr Med J. 2019 Feb 18;32:82. doi: 10.11604/pamj.2019.32.82.18167. eCollection 2019. Pan Afr Med J. 2019. PMID: 31223373 Free PMC article.
-
ERS statement on the multidisciplinary respiratory management of ataxia telangiectasia.Eur Respir Rev. 2015 Dec;24(138):565-81. doi: 10.1183/16000617.0066-2015. Eur Respir Rev. 2015. PMID: 26621971 Free PMC article. Review.
References
References to studies included in this review
Bennoor 2012 {published data only}
-
- Bennoor KS, Afreen KF, Hossain MA, Mahmud AM, Hassan MR. Inhaled mannitol in patients with bronchiectasis: effect on lung function and health status [Abstract]. Respirology 2012;17(Suppl 2):49 [423]. []
Bilton 2013 {published data only}
-
- Safety and efficacy of bronchitol in bronchiectasis. http://ClinicalTrials.gov/show/NCT00277537 (accessed 6 November 2013).
-
- Bilton D, Daviskas E, Anderson SD, Kolbe J, King G, Stirling RG, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non‐cystic fibrosis bronchiectasis. Chest 2013;144(1):215–25. - PubMed
-
- Bilton D, Daviskas E, Jaques A, Anderson S, Charlton B. A randomised placebo‐controlled trial of inhaled mannitol in patients with bronchiectasis [Abstract]. European Respiratory Society Annual Congress, Berlin, Germany, October 4‐8. 2008:[P602]. []
-
- Bilton D, Daviskas E, Jaques A, Anderson SD, Charlton B. A randomised, placebo‐controlled trial of inhaled mannitol in patients with bronchiectasis [Abstract]. American Thoracic Society International Conference, May 15‐20, 2009, San Diego. 2009:A3221 [Poster #E21]. []
-
- Daviskas E. A new approach to treatment of difficult bronchiectasis [Abstract]. Respirology 2007;12(Suppl 4):A99. []
Bradley 2011 {published data only}
-
- Bradley JM, Treacy K, O'Neill B, McCourt F, Green L, Gardner E, et al. A randomised double blind 13 week crossover trial of hypertonic saline (HTS) (6%) vs isotonic saline (ITS) (0.9%) in patients with bronchiectasis [Abstract]. Thorax 2011;66(Suppl 4):A49 [S106]. []
-
- NCT01112410. Hypertonic saline (6%) versus isotonic saline (0.9%) in bronchiectasis. http://ClinicalTrials.gov/show/NCT01112410 (accessed 6 November 2013).
Chandra 2008 {published data only}
-
- Chandra AR, Jones AS, King GG. Effects of inhaled mannitol treatment on airway wall dimensions measured by HRCT in patients with bronchiectasis [Abstract]. American Thoracic Society International Conference, May 16‐21, 2008, Toronto. 2008:Poster #121. []
Daviskas 1999 {published data only}
-
- Daviskas E, Anderson S, Eberl S, Kim Chan H, Bautovich G. Inhalation of dry powder mannitol improves clearance of mucus in patients with bronchiectasis. American Journal of Respiratory and Critical Care Medicine 1999;159(6):1843‐8. - PubMed
Daviskas 2004 {published data only}
-
- Daviskas E, Turton JA, Anderson SD, Young IV, Young IH, Lassig A, et al. A placebo controlled trial with inhaled mannitol improves health related quality of life in patients with bronchiectasis. European Respiratory Journal 2004;24(Suppl 48):707s.
-
- Goldman MD, Daviskas E, Turton JA, Anderson SD. Inhaled mannitol improves lung function assessed by forced oscillation in a placebo controlled trial in patients with bronchiectasis. European Respiratory Journal 2004;24(Suppl 48):470s.
Daviskas 2008 {published data only}
-
- Daviskas E, Anderson SD, Eberl S, Young IH. Effect of increasing doses of mannitol on mucus clearance in patients with bronchiectasis. European Respiratory Journal 2008;31(4):765‐72. [] - PubMed
Kellet 2011 {published data only}
-
- Kellett F, Niven R. Nebulised 7 % hypertonic sodium chloride improves lung function and airway clearance in non cystic fibrosis bronchiectasis [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22. 2010:[P4587]. []
-
- Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respiratory Medicine 2011;105(12):1831‐5. [] - PubMed
Kellett 2005 {unpublished data only}
-
- Kellett F, Niven RM, Redfern J. Double blind trial of hyperosmolar saline as an adjunct to physiotherapy in patients with bronchiectasis. European Respiratory Journal 2001;18(Suppl 33):484s. []
-
- Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respiratory Medicine 2005;99(1):27‐31. - PubMed
NCT00669331 {published data only}
-
- Bilton D, Tino G, Barker A, Chambers D, DeSoyza A, Dupont L, et al. Inhaled mannitol for non‐cystic fibrosis bronchiectasis:Results of a 12 month, multi‐centre, double‐blind, controlled study. ERS Annual Conference September 7‐11 2013, Barcelona. 2013.
-
- NCT00669331. Inhaled mannitol as a mucoactive therapy for bronchiectasis. http://ClinicalTrials.gov/show/NCT00669331 (accessed 6 November 2013).
Nicolson 2012 {published data only}
-
- NCT00484263. The long term effect of inhaled hypertonic saline (6%) in patients with non cystic fibrosis bronchiectasis. http://ClinicalTrials.gov/show/NCT00484263 (accessed 6 November 2013).
-
- Nicolson CHH, Stirling RG, Borg BM, Button BM, Wilson JW, Holland AE. The long term effect of inhaled hypertonic saline 6% in non‐cystic fibrosis bronchiectasis. Respiratory Medicine 2012;106(5):661‐7. [] - PubMed
References to studies excluded from this review
Briffa 2009 {published data only}
-
- Briffa PJ, Anderson SD, Burton DL, Young IH. Prevention of airway narrowing following mannitol inhalation in subjects with bronchiectasis [Abstract]. Respirology 2009;14(Suppl 1):A1. []
Briffa 2011 {published data only}
-
- Briffa PJ, Anderson SD, Burton DL, Young IH. Sodium cromoglycate and eformoterol attenuate sensitivity and reactivity to inhaled mannitol in subjects with bronchiectasis. Respirology 2011;16(1):161‐6. [] - PubMed
Daviskas 2001 {published data only}
-
- Daviskas E, Anderson SD, Eberl S, Chan HK, Young IH. The 24‐h effect of Mannitol on the clearance of mucus in patients with bronchiectasis. Chest 2001;119(2):414‐21. - PubMed
Daviskas 2003 {published data only}
-
- Daviskas E, Anderson SD, Gomes K, Briffa P, Cochrane B, Chan K, et al. Increase in health status after 12 days treatment with inhaled mannitol in patients with bronchiectasis. European Respiratory Journal 2003;22(Suppl 45):430s.
Daviskas 2010 {published data only}
-
- Daviskas E, Anderson SD, Eberl S, Young IH. Beneficial effect of inhaled mannitol and cough in asthmatics with mucociliary dysfunction. Respiratory Medicine 2010;104(11):1645‐53. [] - PubMed
Harrison 1983 {published data only}
-
- Harrison AC, Fleming J, Rea HH, Harris EA. Bronchial hyper‐reactivity in bronchiectasis. Australian and New Zealand Journal of Medicine 1983;13(5):544. [; 4900100000008522]
Murray 2011 {published data only}
-
- Murray MP, Govan JRW, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, et al. A randomized controlled trial of nebulized gentamicin in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2011;183(4):491‐9. [] - PubMed
NCT00105183 {published data only}
-
- NCT00105183. EZ‐2053 in the prophylaxis of acute pulmonary allograft rejection. http://ClinicalTrials.gov/show/NCT00105183 (accessed 6 November 2013).
NCT00730977 {published data only}
-
- NCT00730977. A pilot study to investigate administration of mannitol via a novel dry powder inhaler device. http://ClinicalTrials.gov/show/NCT00730977 Vol. (accessed 6 November 2013).
NCT00749866 {published data only}
-
- NCT00749866. Long term nebulised gentamicin in patients with bronchiectasis. http://ClinicalTrials.gov/show/NCT00749866 (accessed 6 November 2013).
NCT01076491 {published data only}
-
- NCT01076491. High dose inhaled mannitol study. http://ClinicalTrials.gov/show/NCT01076491 Vol. (accessed 6 November 2013).
NCT01313624 {published data only}
-
- NCT01313624. A study to see if AZLI (an inhaled antibiotic) is effective in treating adults with non‐CF bronchiectasis ‐ AIR‐BX1. http://ClinicalTrials.gov/show/NCT01313624 (accessed 6 November 2013).
NCT01314716 {published data only}
-
- NCT01314716. A study to see if AZLI (an inhaled antibiotic) is effective in treating adults with non‐CF bronchiectasis ‐ AIR‐BX2. http://ClinicalTrials.gov/show/NCT01314716 (acessed 6 November 2013).
NCT01677403 {published data only}
-
- NCT01677403. A study to access safety and efficacy of nebulized tobramycin in patients with bronchiectasis. http://clinicaltrials.gov/show/NCT01677403 (accessed 6 November 2013).
Serisier 2013 {published data only}
-
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: The BLESS randomized controlled trial. JAMA 2013;309(12):1260‐7. - PubMed
Additional references
Anwar 2013
-
- Anwar GA, McDonnell MJ, Worthy SA, Bourke SC, Afolabi G, Lordan J, et al. Phenotyping adults with non‐cystic fibrosis bronchiectasis: A prospective observational cohort study. Respiratory Medicine 2013;107(7):1001–7. - PubMed
Bonavita 2012
-
- Bonavita J, Naidich DP. Imaging of bronchiectasis. Clinics in Chest Medicine 2012;33(2):233–48. - PubMed
BTS 2010
-
- Pasteur MC, Bilton D, Hill AT (on behalf of the British Thoracic Society Bronchiectasis (non‐CF) Guideline Group:a sub‐group of the British Thoracic Society Standards of Care Committee). BTS Guideline for non‐CF Bronchiectasis. http://www.brit‐thoracic.org.uk/Guidelines/Bronchiectasis‐Guideline‐non‐CF.aspx (accessed 6 November 2013).
Daviskas 1996
-
- Daviskas E, Anderson SD, Gonda I, Eberl S, Meikle S, Seale JP, et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. European Respiratory Journal 1996;9(4):725‐32. - PubMed
Elkins 2006
-
- Elkins MR, Bye PTP. Inhaled hypertonic saline as a therapy for cystic fibrosis. Current Opinion in Pulmonary Medicine 2006;12(6):445–52. - PubMed
Elkins 2006a
-
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long‐term inhaled hypertonic saline in patients with cystic fibrosis. New England Journal of Medicine 2006;354(3):229‐40. - PubMed
Enderby 2007
Evans 1996
-
- Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. European Respiratory Journal 1996;9:1601‐4. - PubMed
Frey 2007
-
- Frey JG. Bronchiectasis: a reemerging disease. Revue Médicale Suisse 2007;3(99):477‐8, 480‐3. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration, Available from www.cochrane‐handbook.org (accessed 10/12/2013)., updated March 2011.
Jones 2005
-
- Jones PW. St Georges Respiratory Questionnaire:MCID. COPD 2005;2(1):75‐9. - PubMed
Kleinewietfeld 2013
Martinez 2007
-
- Martinez‐García MA, Soler‐Cataluña JJ, Perpiñá‐Tordera M, Román‐Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. - PubMed
Martinez 2013
-
- Martínez‐García M‐A, Rosa Carrillo D, Soler‐Cataluña J‐J, Donat‐Sanz Y, Serra PC, Lerma MA, et al. Prognostic value of bronchiectasis in patients with moderate‐to‐severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187(8):823–31. - PubMed
Pasteur 2010
-
- Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non‐CF bronchiectasis. Thorax 2010;65:Suppl 1:i1‐58. - PubMed
Pavia 1978
-
- Pavia D, Thomson ML, Clarke SW. Enhanced clearance of secretions from the human lung after the administration of hypertonic saline aerosol. American Review of Respiratory Disease 1978;117(2):199‐203. - PubMed
Reeves 2011
-
- Reeves EP, Williamson M, O'Neill SJ, Greally P, McElvaney NG. Nebulized hypertonic saline decreases IL‐8 in sputum of patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1517‐23. - PubMed
Review Manager (RevMan) [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robinson 1997
Shibuya 2003
-
- Shibuya Y, Wills PJ, Cole PJ. Effect of osmolality on mucociliary transportability and rheology of cystic fibrosis and bronchiectasis sputum. Respirology 2003;8(2):181‐5. - PubMed
Shoemark 2007
-
- Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respiratory Medicine 2007;101(6):1163–70. - PubMed
Sly 2013
-
- Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. Risk factors for bronchiectasis in children with cystic fibrosis. New England Journal of Medicine 2013;368(21):1963–70. - PubMed
TSANZ 2010
-
- Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. http://www.lungfoundation.com.au/wp‐content/uploads/2012/06/bronchiectas... (accessed 12 November 2013).
Weycker 2005
-
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205‐9.
Wills 1997
References to other published versions of this review
Wills 2002
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

