Ergosterol concentration and variability in genotype-by-pathogen interaction for grain mold resistance in sorghum
- PMID: 24817586
- PMCID: PMC4107276
- DOI: 10.1007/s00425-014-2081-7
Ergosterol concentration and variability in genotype-by-pathogen interaction for grain mold resistance in sorghum
Abstract
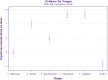
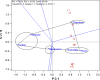
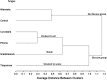
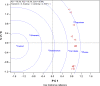
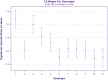
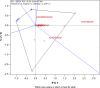
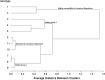
A lack of understanding of host-by-pathogen relations can hinder the success of breeding for resistance to a major disease. Fungal strain pathogenicity has to be understood from the virulence it can cause on susceptible genotypes and host resistance indicates which genotypes have resistance genes. Where the two worlds meet lies the place where researchers match the prevalent pathogen in the area of production with resistant varieties. This paper uses ergosterol concentration analysis as a measure of fungal biomass accumulation to assess levels of resistance in host genotypes. 11 sorghum genotypes were inoculated with 5 strains of fungi that are known to be associated with grain mold disease of sorghum. The resulting interaction was analyzed using GGE Biplot analysis and Cluster analysis which showed that none of the genotypes were resistant to Phoma sorghina and Curvularia lunata. Three genotypes were resistant to Fusarium thapsinum. One fungal strain (Alternaria alternata) does not contribute any significant damage in the grain mold disease. Fusarium graminearum causes very little grain mold disease. There was no correlation between the fungal strains. Visual scoring did not correlate with ergosterol accumulation. Resistance to grain mold in sorghum is shown to be due to vertical or specific resistance genes. Sorghum breeders should, therefore, identify predominant fungal strains in their localities and then locate and tag these resistance genes in their germplasm and pyramid them in commercial varieties.
Figures



Similar articles
-
The Sorghum Grain Mold Disease Complex: Pathogens, Host Responses, and the Bioactive Metabolites at Play.Front Plant Sci. 2021 May 28;12:660171. doi: 10.3389/fpls.2021.660171. eCollection 2021. Front Plant Sci. 2021. PMID: 34122480 Free PMC article. Review.
-
Isolation and characterization of the grain mold fungi Cochliobolus and Alternaria spp. from sorghum using semiselective media and DNA sequence analyses.Can J Microbiol. 2013 Feb;59(2):87-96. doi: 10.1139/cjm-2012-0649. Epub 2012 Dec 6. Can J Microbiol. 2013. PMID: 23461515
-
Expression of pathogenesis-related protein PR-10 in sorghum floral tissues in response to inoculation with Fusarium thapsinum and Curvularia lunata.Mol Plant Pathol. 2010 Jan;11(1):93-103. doi: 10.1111/j.1364-3703.2009.00580.x. Mol Plant Pathol. 2010. PMID: 20078779 Free PMC article.
-
Role of chitinase and sormatin accumulation in the resistance of sorghum cultivars to grain mold.J Agric Food Chem. 2005 Jul 13;53(14):5565-70. doi: 10.1021/jf050367t. J Agric Food Chem. 2005. PMID: 15998115
-
Antifungal proteins and other mechanisms in the control of sorghum stalk rot and grain mold.J Agric Food Chem. 2001 Oct;49(10):4732-42. doi: 10.1021/jf010007f. J Agric Food Chem. 2001. PMID: 11600015 Review.
Cited by
-
Development of Sorghum Genotypes for Improved Yield and Resistance to Grain Mold Using Population Breeding Approach.Front Plant Sci. 2021 Jul 28;12:687332. doi: 10.3389/fpls.2021.687332. eCollection 2021. Front Plant Sci. 2021. PMID: 34394141 Free PMC article.
-
The Sorghum Grain Mold Disease Complex: Pathogens, Host Responses, and the Bioactive Metabolites at Play.Front Plant Sci. 2021 May 28;12:660171. doi: 10.3389/fpls.2021.660171. eCollection 2021. Front Plant Sci. 2021. PMID: 34122480 Free PMC article. Review.
References
-
- Audilakshmi S, Stenhouse JW, Reddy TP, Prasad MVR. Grain mould resistance and associated characters of sorghum genotypes. Euphytica. 1999;107:91–103. doi: 10.1023/A:1026410913896. - DOI
-
- Bandyopadhyay R, Mughogho LK. Evaluation of field screening techniques for resistance to sorghum grain molds. Plant Dis. 1988;72:500–503. doi: 10.1094/PD-72-0500. - DOI
-
- Bandyopadhyay R, Mughogho LK, Satyanarayana MV, Kalisz ME. Occurrence of airborne spores of fungi causing mold over a sorghum crop. Mycol Res. 1991;95:1315–1320. doi: 10.1016/S0953-7562(09)80583-2. - DOI
-
- Castor LL (1981) Grain mold histopathology, damage assessment and resistance screening within Sorghum bicolor (L.) Moench lines. PhD thesis, Texas A&M University, College Station, TX, USA, pp 192
-
- Castor LL, Frederiksen RA (1980) Fusarium and Curvularia grain mold in Texas. In: Wlliams RJ, Frederiksen RA, Mughogho LK, Bengston GD (eds) Sorghum diseases, a world review. Proceedings of an international workshop on sorghum diseases, 11–15 December, 1978. ICRISAT, Hyderabad, India, pp 93–102
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

