Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation
- PMID: 24817693
- PMCID: PMC4040554
- DOI: 10.1073/pnas.1315346111
Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation
Abstract
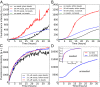
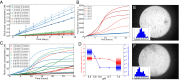
The formation of amyloid fibrils by the intrinsically disordered protein α-synuclein is a hallmark of Parkinson disease. To characterize the microscopic steps in the mechanism of aggregation of this protein we have used in vitro aggregation assays in the presence of preformed seed fibrils to determine the molecular rate constant of fibril elongation under a range of different conditions. We show that α-synuclein amyloid fibrils grow by monomer and not oligomer addition and are subject to higher-order assembly processes that decrease their capacity to grow. We also find that at neutral pH under quiescent conditions homogeneous primary nucleation and secondary processes, such as fragmentation and surface-assisted nucleation, which can lead to proliferation of the total number of aggregates, are undetectable. At pH values below 6, however, the rate of secondary nucleation increases dramatically, leading to a completely different balance between the nucleation and growth of aggregates. Thus, at mildly acidic pH values, such as those, for example, that are present in some intracellular locations, including endosomes and lysosomes, multiplication of aggregates is much faster than at normal physiological pH values, largely as a consequence of much more rapid secondary nucleation. These findings provide new insights into possible mechanisms of α-synuclein aggregation and aggregate spreading in the context of Parkinson disease.
Keywords: electrostatic interactions; kinetic analysis; neurodegenerative disease; prion-like behavior; seeding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Otzen DE, editor. Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties. Germany: Wiley-VCH, Weinheim; 2013.
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Cohen SIA, Vendruscolo M, Dobson CM, Knowles TPJ. From macroscopic measurements to microscopic mechanisms of protein aggregation. J Mol Biol. 2012;421(2-3):160–171. - PubMed
-
- Ferrone FA, Hofrichter J, Eaton WA. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol. 1985;183(4):611–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

