Olfactory Neuron Patterning and Specification
- PMID: 24817923
- PMCID: PMC4015351
- DOI: 10.1016/b978-008045046-9.01046-9
Olfactory Neuron Patterning and Specification
Figures





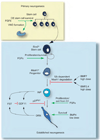
References
-
- Beites CL, Kawauchi S, Crocker CE, et al. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Experimental Cell Research. 2005;306:309–316. - PubMed
-
- Bhattacharyya S, Bronner-Fraser M. Hierarchy of regulatory events in sensory placode development. Current Opinion in Genetics & Development. 2004;14:520–526. - PubMed
-
- Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. - PubMed
-
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. - PubMed
-
- Cau E, Gradwohl G, Fode C, et al. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. - PubMed

