Features of gastric glomus tumor: a clinicopathologic, immunohistochemical and molecular retrospective study
- PMID: 24817939
- PMCID: PMC4014223
Features of gastric glomus tumor: a clinicopathologic, immunohistochemical and molecular retrospective study
Abstract
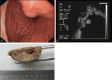
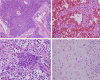
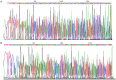
Glomus tumor (GT) of the stomach is a rare mesenchymal tumor. There have been few detailed studies on these tumors. A total of 1894 cases of resected gastric mesenchymal tumors were collected and eleven confirmed gastric GTs were studied. The clinical, pathological, immunohistochemical, ultrastructural and molecular characteristics of the tumors were analyzed through a retrospective study. Histologically, most tumors had gastric smooth muscle immediately adjacent and surrounding the tumor. Tumor cells around blood vessels were small, uniform, and round. Foci of hyaline and myxoid changes were observed. Prominent clear cell features were observed in two tumors. Positive expression of α-smooth muscle actin (α-SMA), laminin, collagen type IV, and vimentin was detected by immunohistochemical analysis in all patients. However, in clear cell areas the expression of α-SMA, laminin, and type IV collagen were mild, while Syn was positive. Moreover, myofibrils and neuroendocrine granules were also present in the cytoplasm of these cells. No C-kit or PDGFR-α genetic mutations were detected in all patients. To conclude, Our results show that GTs in the stomach are histologically and immunophenotypically fully comparable with the glomus tumors of peripheral soft tissues. Neuroendocrine granules and neuroendocrine differentiation were identified in some of the gastric GT cells. Thus, a novel subtype of gastric glomus tumor expressing neuroendocrine cell markers may exist.
Keywords: Glomus tumor; diagnosis; immunohistochemistry; ultrastructure: stomach.
Figures


References
-
- Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301–311. - PubMed
-
- Orellana F, Onetto C, Balbontin P, Videla D, Manriquez L, Plass R, Araya R, Sepulveda R, Saenz R, Rios H. Gastric glomus tumor: report of one case and review. Endoscopy. 2011;43(Suppl 2 UCTN):E71–72. - PubMed
-
- Tsuneyoshi M, Enjoji M. Glomus tumor: a clinicopathologic and electron microscopic study. Cancer. 1982;50:1601–1607. - PubMed
-
- De Cocker J, Messaoudi N, Waelput W, Van Schil PE. Intrapulmonary glomus tumor in a young woman. Interact Cardiovasc Thorac Surg. 2008;7:1191–1193. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
