A new look at cerebrospinal fluid circulation
- PMID: 24817998
- PMCID: PMC4016637
- DOI: 10.1186/2045-8118-11-10
A new look at cerebrospinal fluid circulation
Abstract
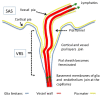
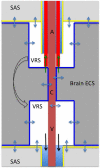
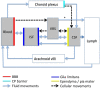
According to the traditional understanding of cerebrospinal fluid (CSF) physiology, the majority of CSF is produced by the choroid plexus, circulates through the ventricles, the cisterns, and the subarachnoid space to be absorbed into the blood by the arachnoid villi. This review surveys key developments leading to the traditional concept. Challenging this concept are novel insights utilizing molecular and cellular biology as well as neuroimaging, which indicate that CSF physiology may be much more complex than previously believed. The CSF circulation comprises not only a directed flow of CSF, but in addition a pulsatile to and fro movement throughout the entire brain with local fluid exchange between blood, interstitial fluid, and CSF. Astrocytes, aquaporins, and other membrane transporters are key elements in brain water and CSF homeostasis. A continuous bidirectional fluid exchange at the blood brain barrier produces flow rates, which exceed the choroidal CSF production rate by far. The CSF circulation around blood vessels penetrating from the subarachnoid space into the Virchow Robin spaces provides both a drainage pathway for the clearance of waste molecules from the brain and a site for the interaction of the systemic immune system with that of the brain. Important physiological functions, for example the regeneration of the brain during sleep, may depend on CSF circulation.
Keywords: Aquaporin; Astrocyte; Blood brain barrier; Cerebrospinal fluid circulation; Virchow Robin space.
Figures
References
-
- Davson H. Formation and drainage of the cerebrospinal fluid. Sci Basis Med Annu Rev. 1966. pp. 238–259. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

