Insights on the role of antimicrobial cuffed endotracheal tubes in preventing transtracheal transmission of VAP pathogens from an in vitro model of microaspiration and microbial proliferation
- PMID: 24818125
- PMCID: PMC4003835
- DOI: 10.1155/2014/120468
Insights on the role of antimicrobial cuffed endotracheal tubes in preventing transtracheal transmission of VAP pathogens from an in vitro model of microaspiration and microbial proliferation
Abstract
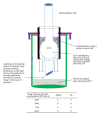
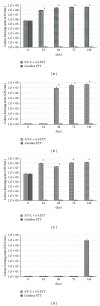
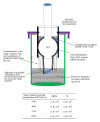
We developed an in vitro model to evaluate the effect of different cuffed endotracheal tubes (ETTs) on transtracheal transmission of ventilator-associated pneumonia (VAP) pathogens along external surfaces of ETTs. The model independently assessed the relative contributions of microbial proliferation to the distal tip and microaspiration of contaminated secretions past the cuff by testing in three modes: microaspiration only, microbial proliferation only, and simultaneous microaspiration and microbial proliferation. We evaluated transmission of methicillin resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa (PA) in the presence of a standard ETT; a soft, tapered cuff ETT with subglottic suctioning; and a novel antimicrobial gendine (combination of gentian violet and chlorhexidine) ETT in the model. In the microaspiration only mode, when leakage past the cuff occurred quickly, no ETT prevented transmission. When microaspiration was delayed, the gendine ETT was able to completely disinfect the fluid above the cuff and thereby prevent transmission of pathogens. In microbial proliferation only mode, the gendine ETT was the sole ETT that prevented transmission. With both mechanisms simultaneously available, transmission was dependent on how long microaspiration was delayed. Potent antimicrobial ETTs, such as a gendine ETT, can make unique contributions to prevent VAP when microaspiration is gradual.
Figures


Similar articles
-
The prevention of biofilm colonization by multidrug-resistant pathogens that cause ventilator-associated pneumonia with antimicrobial-coated endotracheal tubes.Biomaterials. 2011 Apr;32(11):2689-94. doi: 10.1016/j.biomaterials.2010.12.015. Epub 2011 Feb 3. Biomaterials. 2011. PMID: 21295343
-
Randomized Pilot Trial of Two Modified Endotracheal Tubes To Prevent Ventilator-associated Pneumonia.Ann Am Thorac Soc. 2016 Jan;13(1):72-80. doi: 10.1513/AnnalsATS.201506-346OC. Ann Am Thorac Soc. 2016. PMID: 26523433 Free PMC article. Clinical Trial.
-
A rapid method of impregnating endotracheal tubes and urinary catheters with gendine: a novel antiseptic agent.J Antimicrob Chemother. 2005 Jan;55(1):51-6. doi: 10.1093/jac/dkh499. Epub 2004 Dec 1. J Antimicrob Chemother. 2005. PMID: 15574478
-
New endotracheal tubes designed to prevent ventilator-associated pneumonia: do they make a difference?Respir Care. 2010 Aug;55(8):1046-55. Respir Care. 2010. PMID: 20667152 Review.
-
How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients.BMC Infect Dis. 2014 Nov 28;14:119. doi: 10.1186/1471-2334-14-119. BMC Infect Dis. 2014. PMID: 25430629 Free PMC article. Review.
Cited by
-
Molecular analysis of endotracheal tube biofilms and tracheal aspirates in the pediatric intensive care unit.Adv Pediatr Res. 2017 Dec;4(3):14. doi: 10.12715/apr.2017.4.14. Epub 2017 Nov 10. Adv Pediatr Res. 2017. PMID: 29963643 Free PMC article.
References
-
- Anderson DJ, Kirkland KB, Kaye KS, et al. Underresourced hospital infection control and prevention programs: penny wise, pound foolish? Infection Control and Hospital Epidemiology. 2007;28(7):767–773. - PubMed
-
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Critical Care Medicine. 2003;31(5):1312–1317. - PubMed
-
- Chastre J, Fagon J-Y. Ventilator-associated pneumonia. American Journal of Respiratory and Critical Care Medicine. 2002;165(7):867–903. - PubMed
-
- Pneumatikos IA, Dragoumanis CK, Bouros DE. Ventilator-associated pneumonia or endotracheal tube-associated pneumonia?: an approach to the pathogenesis and preventive strategies emphasizing the importance of endotracheal tube. Anesthesiology. 2009;110(3):673–680. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

