Improved hemostasis in hemophilia mice by means of an engineered factor Va mutant
- PMID: 24818532
- PMCID: PMC4161283
- DOI: 10.1111/jth.12489
Improved hemostasis in hemophilia mice by means of an engineered factor Va mutant
Abstract
Background: Factor (F)VIIa-based bypassing not always provides sufficient hemostasis in hemophilia.
Objectives: To investigate the potential of engineered activated factor V (FVa) variants as bypassing agents in hemophilia A.
Methods: Activity of FVa variants was studied in vitro using prothrombinase assays with purified components and in FV- and FVIII-deficient plasma using clotting and thrombin generation assays. In vivo bleed reduction after the tail clip was studied in hemophilia A mice.
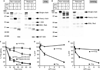
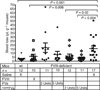
Results and conclusions: FVa mutations included a disulfide bond connecting the A2 and A3 domains and ones that rendered FVa resistant to inactivation by activated protein C (APC). '(super) FVa,' a combination of the A2-A3 disulfide (A2-SS-A3) to stabilize FVa and of APC-cleavage site mutations (Arg506/306/679Gln), had enhanced specific activity and complete APC resistance compared with wild-type FVa, FVL eiden (Arg506Gln), or FVaL eiden (A2-SS-A3). Furthermore, (super) FVa potently increased thrombin generation in vitro in FVIII-deficient plasma. In vivo, (super) FVa reduced bleeding in FVIII-deficient mice more effectively than wild-type FVa. Low-dose (super) FVa, but not wild-type FVa, decreased early blood loss during the first 10 min by more than two-fold compared with saline and provided bleed protection for the majority of mice, similar to treatments with FVIII. During the second 10 min after tail cut, (super) FVa at high dose, but not wild-type FVa, effectively reduced bleeding. These findings suggest that (super) FVa enhances not only clot formation but also clot stabilization. Thus, (super) FVa efficiently improved hemostasis in hemophilia in vitro and in vivo and may have potential therapeutic benefits as a novel bypassing agent in hemophilia.
Keywords: animal experimentation; bleeding; factor V; hemophilia; hemostasis.
© 2013 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The manuscript has been read and approved by all authors. The authors state that the University of California and the Scripps Research Institute have patents in preparation or pending that are related to this report and involve some coauthors.
Figures




Similar articles
-
Safety, Stability and Pharmacokinetic Properties of (super)Factor Va, a Novel Engineered Coagulation Factor V for Treatment of Severe Bleeding.Pharm Res. 2016 Jun;33(6):1517-26. doi: 10.1007/s11095-016-1895-3. Epub 2016 Mar 9. Pharm Res. 2016. PMID: 26960296 Free PMC article.
-
Interdomain engineered disulfide bond permitting elucidation of mechanisms of inactivation of coagulation factor Va by activated protein C.Protein Sci. 2002 Sep;11(9):2091-101. doi: 10.1110/ps.0210002. Protein Sci. 2002. PMID: 12192065 Free PMC article.
-
An engineered factor Va prevents bleeding induced by anticoagulant wt activated protein C.PLoS One. 2014 Aug 15;9(8):e104304. doi: 10.1371/journal.pone.0104304. eCollection 2014. PLoS One. 2014. PMID: 25127130 Free PMC article.
-
Factor V and thrombotic disease: description of a janus-faced protein.Arterioscler Thromb Vasc Biol. 2002 Apr 1;22(4):530-8. doi: 10.1161/01.atv.0000012665.51263.b7. Arterioscler Thromb Vasc Biol. 2002. PMID: 11950687 Review.
-
Regulation of thrombin formation by activated protein C: effect of the factor V Leiden mutation.Semin Hematol. 1997 Jul;34(3):244-55. Semin Hematol. 1997. PMID: 9241709 Review.
Cited by
-
Adeno-associated virus-mediated expression of activated factor V (FVa) for hemophilia phenotypic correction.Front Med (Lausanne). 2022 Aug 5;9:880763. doi: 10.3389/fmed.2022.880763. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35991645 Free PMC article.
-
Protein-Engineered Coagulation Factors for Hemophilia Gene Therapy.Mol Ther Methods Clin Dev. 2018 Dec 31;12:184-201. doi: 10.1016/j.omtm.2018.12.007. eCollection 2019 Mar 15. Mol Ther Methods Clin Dev. 2018. PMID: 30705923 Free PMC article. Review.
-
Acquired hemophilia A: emerging treatment options.J Blood Med. 2015 May 8;6:143-50. doi: 10.2147/JBM.S77332. eCollection 2015. J Blood Med. 2015. PMID: 26056504 Free PMC article. Review.
-
Recent advances in use of fresh frozen plasma, cryoprecipitate, immunoglobulins, and clotting factors for transfusion support in patients with hematologic disease.Semin Hematol. 2020 Apr;57(2):73-82. doi: 10.1053/j.seminhematol.2020.07.006. Epub 2020 Jul 27. Semin Hematol. 2020. PMID: 32892846 Free PMC article. Review.
-
Mechanisms of vascular permeability and remodeling associated with hemarthrosis in factor VIII-deficient mice.J Thromb Haemost. 2019 Nov;17(11):1815-1826. doi: 10.1111/jth.14567. Epub 2019 Aug 9. J Thromb Haemost. 2019. PMID: 31301687 Free PMC article.
References
-
- Hoffman M, Monroe DM, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis. 1998;9(Suppl. 1):S61–S65. - PubMed
-
- van’t Veer C, Mann KG. The regulation of the factor VII-dependent coagulation pathway: rationale for the effectiveness of recombinant factor VIIa in refractory bleeding disorders. Semin Thromb Hemost. 2000;26:367–372. - PubMed
-
- Astermark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, Berntorp E, Group FS. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109:546–551. - PubMed
-
- Hoots WK. Arthropathy in inhibitor patients: differences in the joint status. Semin Hematol. 2008;45:S42–S49. - PubMed
-
- de Paula EV, Kavakli K, Mahlangu J, Ayob Y, Lentz SR, Morfini M, Nemes L, Šalek SZ, Shima M, Windyga J, Ehrenforth S, Chuansumrit A. 1804 (adept(TM)l) Investigators Recombinant factor VIIa analog (vatreptacog alfa [activated]) for treatment of joint bleeds in hemophilia patients with inhibitors: a randomized controlled trial. J Thromb Haemost. 2012;10:81–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

