Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: involvement of CB₁ receptors and TRPV1 channels
- PMID: 24818658
- PMCID: PMC4243976
- DOI: 10.1111/bph.12759
Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: involvement of CB₁ receptors and TRPV1 channels
Abstract
Background and purpose: Palmitoylethanolamide (PEA), a naturally occurring acylethanolamide chemically related to the endocannabinoid anandamide, interacts with targets that have been identified in peripheral nerves controlling gastrointestinal motility, such as cannabinoid CB1 and CB2 receptors, TRPV1 channels and PPARα. Here, we investigated the effect of PEA in a mouse model of functional accelerated transit which persists after the resolution of colonic inflammation (post-inflammatory irritable bowel syndrome).
Experimental approach: Intestinal inflammation was induced by intracolonic administration of oil of mustard (OM). Mice were tested for motility and biochemical and molecular biology changes 4 weeks later. PEA, oleoylethanolamide and endocannabinoid levels were measured by liquid chromatography-mass spectrometry and receptor and enzyme mRNA expression by qRT-PCR.
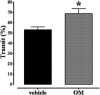
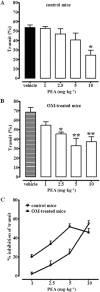
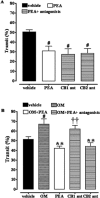
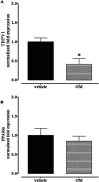
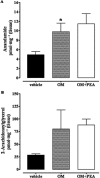
Key results: OM induced transient colitis and a functional post-inflammatory increase in upper gastrointestinal transit, associated with increased intestinal anandamide (but not 2-arachidonoylglycerol, PEA or oleoylethanolamide) levels and down-regulation of mRNA for TRPV1 channels. Exogenous PEA inhibited the OM-induced increase in transit and tended to increase anandamide levels. Palmitic acid had a weaker effect on transit. Inhibition of transit by PEA was blocked by rimonabant (CB1 receptor antagonist), further increased by 5'-iodoresiniferatoxin (TRPV1 antagonist) and not significantly modified by the PPARα antagonist GW6471.
Conclusions and implications: Intestinal endocannabinoids and TRPV1 channel were dysregulated in a functional model of accelerated transit exhibiting aspects of post-inflammatory irritable bowel syndrome. PEA counteracted the accelerated transit, the effect being mediated by CB1 receptors (possibly via increased anandamide levels) and modulated by TRPV1 channels.
© 2014 The British Pharmacological Society.
Figures




Similar articles
-
Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent.Br J Pharmacol. 2015 Jan;172(1):142-58. doi: 10.1111/bph.12907. Epub 2014 Dec 1. Br J Pharmacol. 2015. PMID: 25205418 Free PMC article.
-
Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice.Br J Pharmacol. 2012 Jun;166(4):1444-60. doi: 10.1111/j.1476-5381.2012.01879.x. Br J Pharmacol. 2012. PMID: 22300105 Free PMC article.
-
Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide.Br J Pharmacol. 2013 Mar;168(6):1430-44. doi: 10.1111/bph.12029. Br J Pharmacol. 2013. PMID: 23083124 Free PMC article.
-
Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance.Best Pract Res Clin Endocrinol Metab. 2009 Feb;23(1):33-49. doi: 10.1016/j.beem.2008.10.003. Best Pract Res Clin Endocrinol Metab. 2009. PMID: 19285259 Review.
-
Mechanisms and clinical applications of palmitoylethanolamide (PEA) in the treatment of neuropathic pain.Inflammopharmacology. 2025 Jan;33(1):121-133. doi: 10.1007/s10787-024-01623-8. Epub 2024 Dec 23. Inflammopharmacology. 2025. PMID: 39714723 Review.
Cited by
-
Mutual Links between the Endocannabinoidome and the Gut Microbiome, with Special Reference to Companion Animals: A Nutritional Viewpoint.Animals (Basel). 2022 Jan 31;12(3):348. doi: 10.3390/ani12030348. Animals (Basel). 2022. PMID: 35158670 Free PMC article. Review.
-
The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism.Front Cell Neurosci. 2022 May 11;16:867267. doi: 10.3389/fncel.2022.867267. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35634468 Free PMC article. Review.
-
Palmitoylethanolamide Counteracts Enteric Inflammation and Bowel Motor Dysfunctions in a Mouse Model of Alzheimer's Disease.Front Pharmacol. 2021 Sep 29;12:748021. doi: 10.3389/fphar.2021.748021. eCollection 2021. Front Pharmacol. 2021. PMID: 34658885 Free PMC article.
-
Palmitoylethanolamide counteracts high-fat diet-induced gut dysfunction by reprogramming microbiota composition and affecting tryptophan metabolism.Front Nutr. 2023 Aug 1;10:1143004. doi: 10.3389/fnut.2023.1143004. eCollection 2023. Front Nutr. 2023. PMID: 37599675 Free PMC article.
-
Effect of an Enteroprotective Complementary Feed on Faecal Markers of Inflammation and Intestinal Microbiota Composition in Weaning Puppies.Vet Sci. 2023 Jul 3;10(7):434. doi: 10.3390/vetsci10070434. Vet Sci. 2023. PMID: 37505839 Free PMC article.
References
-
- Abalo R, Cabezos PA, Vera G, Fernández-Pujol R, Martín MI. The cannabinoid antagonist SR144528 enhances the acute effect of WIN 55,212-2 on gastrointestinal motility in the rat. Neurogastroenterol Motil. 2010;22:694. e206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

