Short term feeding of a high fat diet exerts an additive effect on hepatocellular damage and steatosis in liver-specific PTEN knockout mice
- PMID: 24818992
- PMCID: PMC4018288
- DOI: 10.1371/journal.pone.0096553
Short term feeding of a high fat diet exerts an additive effect on hepatocellular damage and steatosis in liver-specific PTEN knockout mice
Abstract
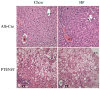
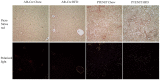
Background: Hepatospecific deletion of PTEN results in constitutive activation of Akt and increased lipogenesis. In mice, the addition of a high fat diet (HFD) downregulates lipogenesis. The aim of this study was to determine the effects of a HFD on hepatocellular damage induced by deletion of PTEN.
Methods: 12 Week old male flox/flox hepatospecific PTEN mice (PTENf/f) or Alb-Cre controls were fed a HFD composed of 45% fat-derived calories (from corn oil) or a normal chow. Animals were then analyzed for hepatocellular damage, oxidative stress and expression of enzymes involved in fatty acid metabolism.
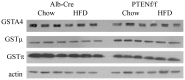
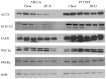
Results: In the Alb-Cre animals, the addition of a HFD resulted in a significant increase in liver triglycerides and altered REDOX capacity as evidenced by increased GPX activity, decreased GST activity and decreased hepatic concentrations of GSSG. In addition, SCD2, ACLY and FASN were all downregulated by the addition of HFD. Furthermore, expression of PPARα and PPARα-dependent proteins Cyp4a and ACSL1 were upregulated. In the PTENf/f mice, HFD resulted in significant increased in ALT, serum triglycerides and decreased REDOX capacity. Although expression of fatty acid synthetic enzymes was elevated in the chow fed PTENf/f group, the addition of HFD resulted in SCD2, ACLY and FASN downregulation. Compared to the Alb-Cre HFD group, expression of PGC1α, PPARα and its downstream targets ACSL and Cyp4a were upregulated in PTENf/f mice.
Conclusions: These data suggest that during conditions of constitutive Akt activation and increased steatosis, the addition of a HFD enhances hepatocellular damage due to increased CD36 expression and altered REDOX status. In addition, this work indicates HFD-induced hepatocellular damage occurs in part, independently of Akt signaling.
Conflict of interest statement
Figures


References
-
- Watanabe S, Horie Y, Suzuki A (2005) Hepatocyte-specific Pten-deficient mice as a novel model for nonalcoholic steatohepatitis and hepatocellular carcinoma. Hepatol Res 33: 161–166. - PubMed
-
- Hashizume H, Sato K, Takagi H, Hirokawa T, Kojima A, et al. (2007) Primary liver cancers with nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol 19: 827–834. - PubMed
-
- Watanabe S, Horie Y, Kataoka E, Sato W, Dohmen T, et al. (2007) Non-alcoholic steatohepatitis and hepatocellular carcinoma: lessons from hepatocyte-specific phosphatase and tensin homolog (PTEN)-deficient mice. J Gastroenterol Hepatol 22 Suppl 1S96–S100. - PubMed
-
- Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, et al. (2007) Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133: 80–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

