Cerebral cortex hyperthyroidism of newborn mct8-deficient mice transiently suppressed by lat2 inactivation
- PMID: 24819605
- PMCID: PMC4018440
- DOI: 10.1371/journal.pone.0096915
Cerebral cortex hyperthyroidism of newborn mct8-deficient mice transiently suppressed by lat2 inactivation
Abstract
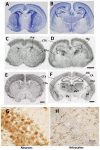
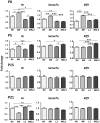
Thyroid hormone entry into cells is facilitated by transmembrane transporters. Mutations of the specific thyroid hormone transporter, MCT8 (Monocarboxylate Transporter 8, SLC16A2) cause an X-linked syndrome of profound neurological impairment and altered thyroid function known as the Allan-Herndon-Dudley syndrome. MCT8 deficiency presumably results in failure of thyroid hormone to reach the neural target cells in adequate amounts to sustain normal brain development. However during the perinatal period the absence of Mct8 in mice induces a state of cerebral cortex hyperthyroidism, indicating increased brain access and/or retention of thyroid hormone. The contribution of other transporters to thyroid hormone metabolism and action, especially in the context of MCT8 deficiency is not clear. We have analyzed the role of the heterodimeric aminoacid transporter Lat2 (Slc7a8), in the presence or absence of Mct8, on thyroid hormone concentrations and on expression of thyroid hormone-dependent cerebral cortex genes. To this end we generated Lat2-/-, and Mct8-/yLat2-/- mice, to compare with wild type and Mct8-/y mice during postnatal development. As described previously the single Mct8 KO neonates had a transient increase of 3,5,3'-triiodothyronine concentration and expression of thyroid hormone target genes in the cerebral cortex. Strikingly the absence of Lat2 in the double Mct8Lat2 KO prevented the effect of Mct8 inactivation in newborns. The Lat2 effect was not observed from postnatal day 5 onwards. On postnatal day 21 the Mct8 KO displayed the typical pattern of thyroid hormone concentrations in plasma, decreased cortex 3,5,3'-triiodothyronine concentration and Hr expression, and concomitant Lat2 inactivation produced little to no modifications. As Lat2 is expressed in neurons and in the choroid plexus, the results support a role for Lat2 in the supply of thyroid hormone to the cerebral cortex during early postnatal development.
Conflict of interest statement
Figures


Similar articles
-
Neuronal 3',3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome.J Neurosci. 2009 Jul 29;29(30):9439-49. doi: 10.1523/JNEUROSCI.6055-08.2009. J Neurosci. 2009. PMID: 19641107 Free PMC article.
-
Changes in thyroid status during perinatal development of MCT8-deficient male mice.Endocrinology. 2013 Jul;154(7):2533-41. doi: 10.1210/en.2012-2031. Epub 2013 May 21. Endocrinology. 2013. PMID: 23696569 Free PMC article.
-
Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells.Glia. 2011 Mar;59(3):463-71. doi: 10.1002/glia.21116. Epub 2010 Dec 29. Glia. 2011. PMID: 21264952
-
Inherited defects of thyroid hormone-cell-membrane transport: review of recent findings.Curr Opin Endocrinol Diabetes Obes. 2013 Oct;20(5):434-40. doi: 10.1097/01.med.0000432531.03233.ad. Curr Opin Endocrinol Diabetes Obes. 2013. PMID: 23974772 Free PMC article. Review.
-
The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models.Biochim Biophys Acta. 2013 Jul;1830(7):3974-8. doi: 10.1016/j.bbagen.2012.04.009. Epub 2012 Apr 20. Biochim Biophys Acta. 2013. PMID: 22543196 Review.
Cited by
-
Involvement of the L-Type Amino Acid Transporter Lat2 in the Transport of 3,3'-Diiodothyronine across the Plasma Membrane.Eur Thyroid J. 2015 Sep;4(Suppl 1):42-50. doi: 10.1159/000381542. Epub 2015 May 28. Eur Thyroid J. 2015. PMID: 26601072 Free PMC article.
-
Thyroid hormone transporters--functions and clinical implications.Nat Rev Endocrinol. 2015 Jul;11(7):406-17. doi: 10.1038/nrendo.2015.66. Epub 2015 May 5. Nat Rev Endocrinol. 2015. PMID: 25942657 Review.
-
Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice.Fluids Barriers CNS. 2015 Sep 4;12:21. doi: 10.1186/s12987-015-0016-8. Fluids Barriers CNS. 2015. PMID: 26337286 Free PMC article.
-
Structural insights into thyroid hormone transport mechanisms of the L-type amino acid transporter 2.Mol Endocrinol. 2015 Jun;29(6):933-42. doi: 10.1210/me.2015-1044. Epub 2015 May 6. Mol Endocrinol. 2015. PMID: 25945809 Free PMC article.
-
Thyroid Hormone Availability and Action during Brain Development in Rodents.Front Cell Neurosci. 2017 Aug 14;11:240. doi: 10.3389/fncel.2017.00240. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28855863 Free PMC article.
References
-
- Visser WE, Friesema EC, Jansen J, Visser TJ (2008) Thyroid hormone transport in and out of cells. Trends Endocrinol Metab 19: 50–56. - PubMed
-
- Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, et al. (2003) Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278: 40128–40135. - PubMed
-
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, et al. (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364: 1435–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

