Biochemical artifacts in experiments involving repeated biopsies in the same muscle
- PMID: 24819751
- PMCID: PMC4098731
- DOI: 10.14814/phy2.286
Biochemical artifacts in experiments involving repeated biopsies in the same muscle
Abstract
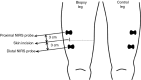
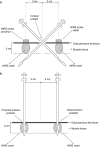
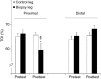
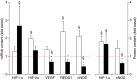
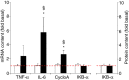
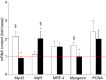
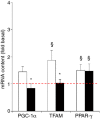
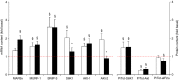
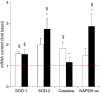
Needle biopsies are being extensively used in clinical trials addressing muscular adaptation to exercise and diet. Still, the potential artifacts due to biopsy sampling are often overlooked. Healthy volunteers (n = 9) underwent two biopsies through a single skin incision in a pretest. Two days later (posttest) another biopsy was taken 3 cm proximally and 3 cm distally to the pretest incision. Muscle oxygenation status (tissue oxygenation index [TOI]) was measured by near-infrared spectroscopy. Biopsy samples were analyzed for 40 key markers (mRNA and protein contents) of myocellular O2 sensing, inflammation, cell proliferation, mitochondrial biogenesis, protein synthesis and breakdown, oxidative stress, and energy metabolism. In the pretest, all measurements were identical between proximal and distal biopsies. However, compared with the pretest, TOI in the posttest was reduced in the proximal (-10%, P < 0.05), but not in the distal area. Conversely, most inflammatory markers were upregulated at the distal (100-500%, P < 0.05), but not at the proximal site. Overall, 29 of the 40 markers measured, equally distributed over all pathways studied, were either up- or downregulated by 50-500% (P < 0.05). In addition, 19 markers yielded conflicting results between the proximal and distal measurements (P < 0.05). This study clearly documents that prior muscle biopsies can cause major disturbances in myocellular signaling pathways in needle biopsies specimens sampled 48 h later. In addition, different biopsy sites within identical experimental conditions yielded conflicting results.
Keywords: Biochemistry; exercise; gene expression; muscle oxygenation; needle biopsy; protein expression; skeletal muscle.
Figures

References
-
- Ameln H., Gustafsson T., Sundberg C. J., Okamoto K., Jansson E., Poellinger L. 2005. Physiological activation of hypoxia inducible factor‐1 in human skeletal muscle. FASEB J.; 19:1009-1011 - PubMed
-
- Bergstrom J. 1963. Clinical studies on cell electrolytes. Scand. J. Clin. Lab. Invest.; 15Suppl. 76:16-18 - PubMed
-
- Bergstrom J. 1975. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest.; 35:609-616 - PubMed
-
- Bokoch G. M., Knaus U. G. 2003. NADPH oxidases: not just for leukocytes anymore!. Trends Biochem. Sci.; 28:502-508 - PubMed
-
- Boushel R., Langberg H., Olesen J., Gonzales‐Alonzo J., Bulow J., Kjaer M. 2001. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand. J. Med. Sci. Sports; 11:213-222 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

