Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A
- PMID: 24819773
- PMCID: PMC4018253
- DOI: 10.1371/journal.pone.0097088
Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A
Abstract
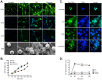
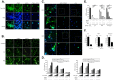
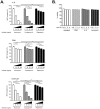
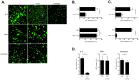
Excessive or aberrant generation of neutrophil extracellular traps (NETs) has recently become implicated in the underlying aetiology of a number of human pathologies including preeclampsia, systemic lupus erythromatosus, rheumatoid arthritis, auto-antibody induced small vessel vasculitis, coagulopathies such as deep vein thrombosis or pulmonary complications. These results imply that effective pharmacological therapeutic strategies will need to be developed to counter overt NETosis in these and other inflammatory disorders. As calcium flux is implicated in the generation of reactive oxygen species and histone citrullination, two key events in NETosis, we analysed the roles of both extra- and intracellular calcium pools and their modulation by pharmacological agents in the NETotic process in detail. Interleukin-8 (IL-8) was used as a physiological stimulus of NETosis. Our data demonstrate that efficient induction of NETosis requires mobilisation of both extracellular and intracellular calcium pools. Since modulation of the calcineurin pathway by cyclosporine A has been described in neutrophils, we investigated its influence on NETosis. Our data indicate that IL-8 induced NETosis is reduced by ascomycin and cyclosporine A, antagonists of the calcineurin pathway, but not following treatment with rapamycin, which utilizes the mTOR pathway. The action of the G protein coupled receptor phospholipase C pathway appears to be essential for the induction of NETs by IL-8, as NETosis was diminished by treatment with either pertussis toxin, a G-protein inhibitor, the phospholipase C inhibitor, U73122, or staurosporine, an inhibitor of protein kinase C. The data regarding the calcineurin antagonists, ascomycin and cyclosporine A, open the possibility to therapeutically suppress or modulate NETosis. They also provide new insight into the mechanism whereby such immune suppressive drugs render transplant patients susceptible to opportunistic fungal infections.
Conflict of interest statement
Figures
Similar articles
-
Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo.J Biol Chem. 2017 May 26;292(21):8616-8629. doi: 10.1074/jbc.M116.768309. Epub 2017 Apr 13. J Biol Chem. 2017. PMID: 28408624 Free PMC article.
-
Histone Acetylation Promotes Neutrophil Extracellular Trap Formation.Biomolecules. 2019 Jan 18;9(1):32. doi: 10.3390/biom9010032. Biomolecules. 2019. PMID: 30669408 Free PMC article.
-
2-Chlorofatty acids: lipid mediators of neutrophil extracellular trap formation.J Lipid Res. 2018 Aug;59(8):1424-1432. doi: 10.1194/jlr.M084731. Epub 2018 May 8. J Lipid Res. 2018. PMID: 29739865 Free PMC article.
-
Post-Translational Modifications in NETosis and NETs-Mediated Diseases.Biomolecules. 2019 Aug 14;9(8):369. doi: 10.3390/biom9080369. Biomolecules. 2019. PMID: 31416265 Free PMC article. Review.
-
The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis.Front Immunol. 2020 Sep 16;11:1749. doi: 10.3389/fimmu.2020.01749. eCollection 2020. Front Immunol. 2020. PMID: 33042107 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps and neutrophilic dermatosis: an update review.Cell Death Discov. 2024 Jan 10;10(1):18. doi: 10.1038/s41420-023-01787-2. Cell Death Discov. 2024. PMID: 38195543 Free PMC article. Review.
-
Preeclampsia - will orphan drug status facilitate innovative biological therapies?Front Surg. 2015 Feb 26;2:7. doi: 10.3389/fsurg.2015.00007. eCollection 2015. Front Surg. 2015. PMID: 25767802 Free PMC article. Review.
-
"The NET Outcome": Are Neutrophil Extracellular Traps of Any Relevance to the Pathophysiology of Autoimmune Disorders in Childhood?Front Pediatr. 2016 Sep 13;4:97. doi: 10.3389/fped.2016.00097. eCollection 2016. Front Pediatr. 2016. PMID: 27679792 Free PMC article. Review.
-
Cathelicidin-mediated lipopolysaccharide signaling via intracellular TLR4 in colonic epithelial cells evokes CXCL8 production.Gut Microbes. 2020 Nov 9;12(1):1785802. doi: 10.1080/19490976.2020.1785802. Epub 2020 Jul 13. Gut Microbes. 2020. PMID: 32658599 Free PMC article.
-
Nitric oxide and peroxynitrite trigger and enhance release of neutrophil extracellular traps.Cell Mol Life Sci. 2020 Aug;77(15):3059-3075. doi: 10.1007/s00018-019-03331-x. Epub 2019 Oct 24. Cell Mol Life Sci. 2020. PMID: 31650185 Free PMC article.
References
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535. - PubMed
-
- Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S (2005) Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol 66: 1146–1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

