Human osteoarthritic cartilage shows reduced in vivo expression of IL-4, a chondroprotective cytokine that differentially modulates IL-1β-stimulated production of chemokines and matrix-degrading enzymes in vitro
- PMID: 24819779
- PMCID: PMC4018406
- DOI: 10.1371/journal.pone.0096925
Human osteoarthritic cartilage shows reduced in vivo expression of IL-4, a chondroprotective cytokine that differentially modulates IL-1β-stimulated production of chemokines and matrix-degrading enzymes in vitro
Erratum in
- PLoS One. 2014;9(8):e105819
Abstract
Background: In osteoarthritis (OA), an inflammatory environment is responsible for the imbalance between the anabolic and catabolic activity of chondrocytes and, thus, for articular cartilage derangement. This study was aimed at providing further insight into the impairment of the anabolic cytokine IL-4 and its receptors in human OA cartilage, as well as the potential ability of IL-4 to antagonize the catabolic phenotype induced by IL-1β.
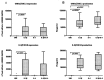
Methodology/principal findings: The in vivo expression of IL-4 and IL-4 receptor subunits (IL-4R, IL-2Rγ, IL-13Rα1) was investigated on full thickness OA or normal knee cartilage. IL-4 expression was found to be significantly lower in OA, both in terms of the percentage of positive cells and the amount of signal per cell. IL-4 receptor type I and II were mostly expressed in mid-deep cartilage layers. No significant difference for each IL-4 receptor subunit was noted. IL-4 anti-inflammatory and anti-catabolic activity was assessed in vitro in the presence of IL-1β and/or IL-4 for 24 hours using differentiated high density primary OA chondrocyte also exhibiting the three IL-4 R subunits found in vivo. Chemokines, extracellular matrix degrading enzymes and their inhibitors were evaluated at mRNA (real time PCR) and protein (ELISA or western blot) levels. IL-4 did not affect IL-1β-induced mRNA expression of GRO-α/CXCL1, IL-8/CXCL8, ADAMTS-5, TIMP-1 or TIMP-3. Conversely, IL-4 significantly inhibited RANTES/CCL5, MIP-1α/CCL3, MIP-1β/CCL4, MMP-13 and ADAMTS-4. These results were confirmed at protein level for RANTES/CCL5 and MMP-13.
Conclusions/significance: Our results indicate for the first time that OA cartilage has a significantly lower expression of IL-4. Furthermore, we found differences in the spectrum of biological effects of IL-4. The findings that IL-4 has the ability to hamper the IL-1β-induced release of both MMP-13 and CCL5/RANTES, both markers of OA chondrocytes, strongly indicates IL-4 as a pivotal anabolic cytokine in cartilage whose impairment impacts on OA pathogenesis.
Conflict of interest statement
Figures






Similar articles
-
Arthropod steroid hormone (20-Hydroxyecdysone) suppresses IL-1β-induced catabolic gene expression in cartilage.BMC Complement Altern Med. 2015 Jan 24;15:1. doi: 10.1186/s12906-015-0520-z. BMC Complement Altern Med. 2015. PMID: 25617057 Free PMC article.
-
MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1β-induced catabolic effects in human chondrocytes.Arthritis Rheum. 2013 Dec;65(12):3141-52. doi: 10.1002/art.38188. Arthritis Rheum. 2013. PMID: 24022470
-
MicroRNA-558 regulates the expression of cyclooxygenase-2 and IL-1β-induced catabolic effects in human articular chondrocytes.Osteoarthritis Cartilage. 2013 Jul;21(7):981-9. doi: 10.1016/j.joca.2013.04.012. Epub 2013 Apr 20. Osteoarthritis Cartilage. 2013. PMID: 23611898
-
Role of Apoptosis in the Pathogenesis of Osteoarthritis: An Explicative Review.Curr Rheumatol Rev. 2024;20(1):2-13. doi: 10.2174/1573397119666230904150741. Curr Rheumatol Rev. 2024. PMID: 37670694 Review.
-
Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism.Ann Rheum Dis. 2008 Dec;67 Suppl 3(0 3):iii75-82. doi: 10.1136/ard.2008.098764. Ann Rheum Dis. 2008. PMID: 19022820 Free PMC article. Review.
Cited by
-
Network-based modelling of mechano-inflammatory chondrocyte regulation in early osteoarthritis.Front Bioeng Biotechnol. 2023 Feb 3;11:1006066. doi: 10.3389/fbioe.2023.1006066. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36815875 Free PMC article.
-
NOTCH1: A Novel Player in the Molecular Crosstalk Underlying Articular Chondrocyte Protection by Oleuropein and Hydroxytyrosol.Int J Mol Sci. 2023 Mar 18;24(6):5830. doi: 10.3390/ijms24065830. Int J Mol Sci. 2023. PMID: 36982904 Free PMC article.
-
Comparative analysis of signaling pathways in peripheral blood from patients with Kashin-Beck disease and osteoarthritis.Exp Ther Med. 2016 Dec;12(6):4077-4084. doi: 10.3892/etm.2016.3879. Epub 2016 Nov 7. Exp Ther Med. 2016. PMID: 28101186 Free PMC article.
-
Interleukin 13 (IL-13)-regulated expression of the chondroprotective metalloproteinase ADAM15 is reduced in aging cartilage.Osteoarthr Cartil Open. 2020 Dec;2(4):100128. doi: 10.1016/j.ocarto.2020.100128. Osteoarthr Cartil Open. 2020. PMID: 33381768 Free PMC article.
-
Ex vivo physiological compression of human osteoarthritis cartilage modulates cellular and matrix components.PLoS One. 2019 Sep 24;14(9):e0222947. doi: 10.1371/journal.pone.0222947. eCollection 2019. PLoS One. 2019. PMID: 31550275 Free PMC article.
References
-
- Borzi RM, Mazzetti I, Marcu KB, Facchini A (2004) Chemokines in cartilage degradation. Clin Orthop Relat Res: S53–61. - PubMed
-
- Huang K, Wu LD (2008) Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res 36: 1149–1160. - PubMed
-
- Donnelly RP, Fenton MJ, Kaufman JD, Gerrard TL (1991) IL-1 expression in human monocytes is transcriptionally and posttranscriptionally regulated by IL-4. J Immunol 146: 3431–3436. - PubMed
-
- Mijatovic T, Kruys V, Caput D, Defrance P, Huez G (1997) Interleukin-4 and -13 inhibit tumor necrosis factor-alpha mRNA translational activation in lipopolysaccharide-induced mouse macrophages. J Biol Chem 272: 14394–14398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

