Understanding brain networks and brain organization
- PMID: 24819881
- PMCID: PMC4157099
- DOI: 10.1016/j.plrev.2014.03.005
Understanding brain networks and brain organization
Abstract
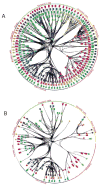
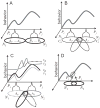
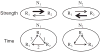
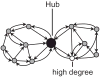
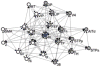
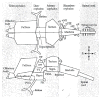
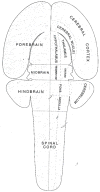
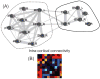
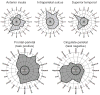
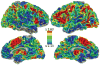
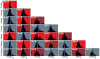
What is the relationship between brain and behavior? The answer to this question necessitates characterizing the mapping between structure and function. The aim of this paper is to discuss broad issues surrounding the link between structure and function in the brain that will motivate a network perspective to understanding this question. However, as others in the past, I argue that a network perspective should supplant the common strategy of understanding the brain in terms of individual regions. Whereas this perspective is needed for a fuller characterization of the mind-brain, it should not be viewed as panacea. For one, the challenges posed by the many-to-many mapping between regions and functions is not dissolved by the network perspective. Although the problem is ameliorated, one should not anticipate a one-to-one mapping when the network approach is adopted. Furthermore, decomposition of the brain network in terms of meaningful clusters of regions, such as the ones generated by community-finding algorithms, does not by itself reveal "true" subnetworks. Given the hierarchical and multi-relational relationship between regions, multiple decompositions will offer different "slices" of a broader landscape of networks within the brain. Finally, I described how the function of brain regions can be characterized in a multidimensional manner via the idea of diversity profiles. The concept can also be used to describe the way different brain regions participate in networks.
Keywords: Brain; Function; Networks; Structure.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures




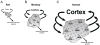


Comment in
-
Dynamic connectivity and dynamic affiliation. Comment on "Understanding brain networks and brain organization" by L. Pessoa.Phys Life Rev. 2014 Sep;11(3):460-1. doi: 10.1016/j.plrev.2014.05.003. Epub 2014 May 21. Phys Life Rev. 2014. PMID: 24874271 Free PMC article. No abstract available.
-
Neural murmurations. Comment on "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):452-4. doi: 10.1016/j.plrev.2014.05.001. Epub 2014 May 16. Phys Life Rev. 2014. PMID: 24890448 No abstract available.
-
Is the brain a decomposable or nondecomposable system? Comment on "Understanding brain networks and brain organization" by Pessoa.Phys Life Rev. 2014 Sep;11(3):458-9. doi: 10.1016/j.plrev.2014.06.005. Epub 2014 Jun 6. Phys Life Rev. 2014. PMID: 24948516 No abstract available.
-
Why network neuroscience? Compelling evidence and current frontiers. Comment on "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):455-7. doi: 10.1016/j.plrev.2014.06.006. Epub 2014 Jun 6. Phys Life Rev. 2014. PMID: 24954730 No abstract available.
-
Brain networks: the next steps. Comment on: "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):440-1. doi: 10.1016/j.plrev.2014.06.014. Epub 2014 Jun 16. Phys Life Rev. 2014. PMID: 24957289 No abstract available.
-
The function of neurocognitive networks. Comment on "Understanding brain networks and brain organization" by Pessoa.Phys Life Rev. 2014 Sep;11(3):438-9. doi: 10.1016/j.plrev.2014.06.004. Epub 2014 Jun 6. Phys Life Rev. 2014. PMID: 24969659 No abstract available.
-
The elusive concept of brain network. Comment on "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):448-51. doi: 10.1016/j.plrev.2014.06.019. Epub 2014 Jun 24. Phys Life Rev. 2014. PMID: 24998043 Free PMC article. No abstract available.
-
Beyond localized and distributed accounts of brain functions. Comment on "Understanding brain networks and brain organization" by Pessoa.Phys Life Rev. 2014 Sep;11(3):442-3. doi: 10.1016/j.plrev.2014.06.018. Epub 2014 Jun 23. Phys Life Rev. 2014. PMID: 24998341 No abstract available.
-
Brain networks and their origins. Comment on "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):444-5. doi: 10.1016/j.plrev.2014.06.022. Epub 2014 Jul 1. Phys Life Rev. 2014. PMID: 25011408 No abstract available.
-
Complex function in the dynamic brain: Comment on "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):436-7. doi: 10.1016/j.plrev.2014.06.017. Epub 2014 Jun 23. Phys Life Rev. 2014. PMID: 25022869 No abstract available.
-
Brain network science needs to become predictive. Comment on "Understanding brain networks and brain organization" by Luiz Pessoa.Phys Life Rev. 2014 Sep;11(3):446-7. doi: 10.1016/j.plrev.2014.07.002. Epub 2014 Jul 8. Phys Life Rev. 2014. PMID: 25066462 No abstract available.
-
Brain networks: moving beyond graphs. Reply to comments on "Understanding brain networks and brain organization".Phys Life Rev. 2014 Sep;11(3):462-6. doi: 10.1016/j.plrev.2014.07.005. Epub 2014 Jul 22. Phys Life Rev. 2014. PMID: 25086837 No abstract available.
References
-
- Shallice T. From neuropsychology to mental structure. New York: Cambridge University Press; 1988.
-
- Pessoa L. The Cognitive-Emotional Brain: From Interactions to Integration. Cambridge: MIT Press; 2013.
-
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–58. - PubMed
-
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annual review of neuroscience. 2010;33:269–98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

