Chromophore chemistry of fluorescent proteins controlled by light
- PMID: 24819887
- PMCID: PMC4096052
- DOI: 10.1016/j.cbpa.2014.04.010
Chromophore chemistry of fluorescent proteins controlled by light
Abstract
Recent progress in molecular engineering of genetically encoded probes whose spectral properties are controlled with light, such as photoactivatable, photoswitchable and reversibly switchable fluorescent proteins, has brought the new possibilities to bioimaging and super-resolution microscopy. The development of modern photoconvertible proteins is linked to the studies of light-induced chromophore transformations. Here, we summarize the current view on the chromophore chemistry in the photocontrollable fluorescent proteins. We describe both the fundamental principles and the specific molecular mechanisms underlying the irreversible and reversible chromophore photoconversions. We discuss advancements in super-resolution microscopy that became possible due to the engineering of new protein phenotypes and understanding of their chromophore transformations.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


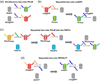
References
-
- Betzig E, Patterson GH, Sougrat R, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
-
-
Grotjohann T, Testa I, Leutenegger M, et al. Diffraction-unlimited all-optical imaging and writing with a photochromic GFP. Nature. 2011;478:204–208. The paper reports the reversibly switchable variant of EGFP with a high fatigue resistance. rsEGFP was used in super-resolution RESOLFT microscopy of live cells.
-
-
-
Subach FV, Zhang L, Gadella TW, et al. Red fluorescent protein with reversibly photoswitchable absorbance for photochromic FRET. Chem Biol. 2010;17:745–755. Engineered in this paper rsTagRFP is the only red reversily switchable fluorescent protein, which changes its absoption upon switching. This property enabled its use in photochromic fluorescence resonance energy transfer (pcFRET).
-
-
-
Subach OM, Patterson GH, Ting LM, et al. A photoswitchable orange-to-far-red fluorescent protein, PSmOrange. Nat Methods. 2011;8:771–777. This paper reports the first orange-to-far-red PSFP and uncoveres the nature of its unique far-red chromophore. The applications of PSmOrange include photolabeling in vivo, multicolor imaging and super-resolution microscopy.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

