Molecular evolution of the primate α-/θ-defensin multigene family
- PMID: 24819937
- PMCID: PMC4018336
- DOI: 10.1371/journal.pone.0097425
Molecular evolution of the primate α-/θ-defensin multigene family
Abstract
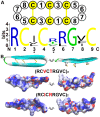
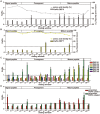
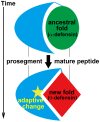
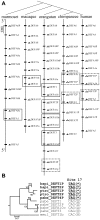
The primate α-/θ-defensin multigene family encodes versatile endogenous cationic and amphipathic peptides that have broad-spectrum antibacterial, antifungal and antiviral activity. Although previous studies have reported that α-/θ-defensin (DEFA/DEFT) genes are under birth-and-death evolution with frequent duplication and rapid evolution, the phylogenetic relationships of the primate DEFA/DEFT genes; the genetic bases for the existence of similar antimicrobial spectra among closely related species; and the evolutionary processes involved in the emergence of cyclic θ-defensins in Old World monkeys and their subsequent loss of function in humans, chimpanzees and gorillas require further investigation. In this study, the DEFA/DEFT gene repertoires from primate and treeshrew were collected, followed by detailed phylogenetic, sequence and structure, selection pressure and comparative genomics analyses. All treeshrew, prosimian and simian DEFA/DEFT genes are grouped into two major clades, which are tissue-specific for enteric and myeloid defensins in simians. The simian enteric and myeloid α-defensins are classified into six functional gene clusters with diverged sequences, variable structures, altered functional constraints and different selection pressures, which likely reflect the antimicrobial spectra among closely related species. Species-specific duplication or pseudogenization within each simian cluster implies that the antimicrobial spectrum is ever-shifting, most likely challenged by the ever-changing pathogen environment. The DEFT evolved from the myeloid DEFA8. The prosegment of θ-defensin is detected with adaptive changes coevolving with the new protein fold of mature peptide, coincident with the importance of the prosegment for the correct folding of the mature peptide. Lastly, a less-is-hitchhiking hypothesis was proposed as a possible explanation for the expansion of pseudogene DEFTP and the loss of functional DEFT, where the gain or loss of the hitchhiker is determined by its adjacent driver gene during the birth-and-death evolutionary process.
Conflict of interest statement
Figures



Similar articles
-
Comparative genomics and evolution of the alpha-defensin multigene family in primates.Mol Biol Evol. 2010 Oct;27(10):2333-43. doi: 10.1093/molbev/msq118. Epub 2010 May 9. Mol Biol Evol. 2010. PMID: 20457584 Free PMC article.
-
Evolution of primate α and θ defensins revealed by analysis of genomes.Mol Biol Rep. 2014 Jun;41(6):3859-66. doi: 10.1007/s11033-014-3253-z. Epub 2014 Feb 21. Mol Biol Rep. 2014. PMID: 24557891
-
Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes.Physiol Genomics. 2004 Dec 15;20(1):1-11. doi: 10.1152/physiolgenomics.00150.2004. Epub 2004 Oct 19. Physiol Genomics. 2004. PMID: 15494476
-
Theta-defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins.Curr Protein Pept Sci. 2004 Oct;5(5):365-71. doi: 10.2174/1389203043379459. Curr Protein Pept Sci. 2004. PMID: 15544531 Review.
-
Rapid sequence divergence in mammalian beta-defensins by adaptive evolution.Mol Immunol. 2003 Nov;40(7):413-21. doi: 10.1016/s0161-5890(03)00160-3. Mol Immunol. 2003. PMID: 14568387 Review.
Cited by
-
Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease.BBA Clin. 2016 Jan 8;5:66-71. doi: 10.1016/j.bbacli.2015.12.003. eCollection 2016 Jun. BBA Clin. 2016. PMID: 27051591 Free PMC article.
-
Designing an optimized theta-defensin peptide for HIV therapy using in-silico approaches.J Integr Bioinform. 2025 Mar 19;22(1):20230053. doi: 10.1515/jib-2023-0053. eCollection 2025 Mar 1. J Integr Bioinform. 2025. PMID: 40098445 Free PMC article.
-
Killing of Staphylococcus aureus and Salmonella enteritidis and neutralization of lipopolysaccharide by 17-residue bovine lactoferricins: improved activity of Trp/Ala-containing molecules.Sci Rep. 2017 Mar 13;7:44278. doi: 10.1038/srep44278. Sci Rep. 2017. PMID: 28287172 Free PMC article.
-
Retrocyclins neutralize bacterial toxins by potentiating their unfolding.Biochem J. 2015 Apr 15;467(2):311-20. doi: 10.1042/BJ20150049. Biochem J. 2015. PMID: 25670244 Free PMC article.
-
Identification of putative orthologs of clinically relevant antimicrobial peptides in the equine ocular surface and amniotic membrane.Vet Ophthalmol. 2023 Apr;26 Suppl 1(Suppl 1):125-133. doi: 10.1111/vop.13042. Epub 2022 Dec 7. Vet Ophthalmol. 2023. PMID: 36478371 Free PMC article.
References
-
- Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3: 710–720. - PubMed
-
- Lehrer RI (2004) Primate defensins. Nat Rev Microbiol 2: 727–738. - PubMed
-
- Selsted ME, Ouellette AJ (2005) Mammalian defensins in the antimicrobial immune response. Nat Immunol 6: 551–557. - PubMed
-
- Klotman ME, Chang TL (2006) Defensins in innate antiviral immunity. Nat Rev Immunol 6: 447–456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

