Plastidial Expression of Type II NAD(P)H Dehydrogenase Increases the Reducing State of Plastoquinones and Hydrogen Photoproduction Rate by the Indirect Pathway in Chlamydomonas reinhardtii1
- PMID: 24820024
- PMCID: PMC4081341
- DOI: 10.1104/pp.114.240432
Plastidial Expression of Type II NAD(P)H Dehydrogenase Increases the Reducing State of Plastoquinones and Hydrogen Photoproduction Rate by the Indirect Pathway in Chlamydomonas reinhardtii1
Abstract
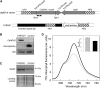
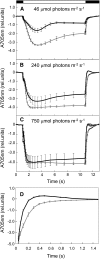
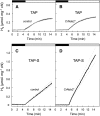
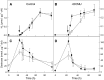
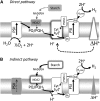
Biological conversion of solar energy into hydrogen is naturally realized by some microalgae species due to a coupling between the photosynthetic electron transport chain and a plastidial hydrogenase. While promising for the production of clean and sustainable hydrogen, this process requires improvement to be economically viable. Two pathways, called direct and indirect photoproduction, lead to sustained hydrogen production in sulfur-deprived Chlamydomonas reinhardtii cultures. The indirect pathway allows an efficient time-based separation of O2 and H2 production, thus overcoming the O2 sensitivity of the hydrogenase, but its activity is low. With the aim of identifying the limiting step of hydrogen production, we succeeded in overexpressing the plastidial type II NAD(P)H dehydrogenase (NDA2). We report that transplastomic strains overexpressing NDA2 show an increased activity of nonphotochemical reduction of plastoquinones (PQs). While hydrogen production by the direct pathway, involving the linear electron flow from photosystem II to photosystem I, was not affected by NDA2 overexpression, the rate of hydrogen production by the indirect pathway was increased in conditions, such as nutrient limitation, where soluble electron donors are not limiting. An increased intracellular starch was observed in response to nutrient deprivation in strains overexpressing NDA2. It is concluded that activity of the indirect pathway is limited by the nonphotochemical reduction of PQs, either by the pool size of soluble electron donors or by the PQ-reducing activity of NDA2 in nutrient-limited conditions. We discuss these data in relation to limitations and biotechnological improvement of hydrogen photoproduction in microalgae.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures
References
-
- Allen JF, Bennett J, Steinback KE, Arntzen CJ. (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29
-
- Alric J. (2010) Cyclic electron flow around photosystem I in unicellular green algae. Photosynth Res 106: 47–56 - PubMed
-
- Alric J, Lavergne J, Rappaport F. (2010) Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii (I) aerobic conditions. Biochim Biophys Acta 1797: 44–51 - PubMed
-
- Antal TK, Krendeleva TE, Laurinavichene TV, Makarova VV, Ghirardi ML, Rubin AB, Tsygankov AA, Seibert M. (2003) The dependence of algal H2 production on photosystem II and O2 consumption activities in sulfur-deprived Chlamydomonas reinhardtii cells. Biochim Biophys Acta 1607: 153–160 - PubMed
-
- Antal TK, Volgusheva AA, Kukarskih GP, Krendeleva TE, Rubin AB. (2009) Relationships between H2 photoproduction and different electron transport pathways in sulfur-deprived Chlamydomonas reinhardtii. Int J Hydrogen Energy 34: 9087–9094
LinkOut - more resources
Full Text Sources
Other Literature Sources

