The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells
- PMID: 24820173
- PMCID: PMC4018389
- DOI: 10.1371/journal.pone.0096778
The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells
Abstract
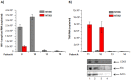
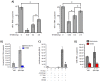
HIV-1 infection results in a chronic but incurable illness since long-term HAART can keep the virus to an undetectable level. However, discontinuation of therapy rapidly increases viral burden. Moreover, patients under HAART frequently develop various metabolic disorders and HIV-associated neuronal disease. Today, the main challenge of HIV-1 research is the elimination of the residual virus in infected individuals. The current HIV-1 diagnostics are largely comprised of serological and nucleic acid based technologies. Our goal is to integrate the nanotrap technology into a standard research tool that will allow sensitive detection of HIV-1 infection. This study demonstrates that majority of HIV-1 virions in culture supernatants and Tat/Nef proteins spiked in culture medium can be captured by nanotrap particles. To determine the binding affinities of different baits, we incubated target molecules with nanotrap particles at room temperature. After short sequestration, materials were either eluted or remained attached to nanotrap particles prior to analysis. The unique affinity baits of nanotrap particles preferentially bound HIV-1 materials while excluded albumin. A high level capture of Tat or Tat peptide by NT082 and NT084 particles was measured by western blot (WB). Intracellular Nef protein was captured by NT080, while membrane-associated Nef was captured by NT086 and also detected by WB. Selective capture of HIV-1 particles by NT073 and NT086 was measured by reverse transcriptase assay, while capture of infectious HIV-1 by these nanoparticles was demonstrated by functional transactivation in TZM-bl cells. We also demonstrated specific capture of HIV-1 particles and exosomes-containing TAR-RNA in patients' serum by NT086 and NT082 particles, respectively, using specific qRT-PCR. Collectively, our data indicate that certain types of nanotrap particles selectively capture specific HIV-1 molecules, and we propose to use this technology as a platform to enhance HIV-1 detection by concentrating viral proteins and infectious virions from infected samples.
Conflict of interest statement
Figures


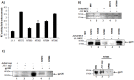


References
-
- Fields BN KD, Howley PM, et al.. (2013) Fields Virology 6th Ed - Human Immunodeficiency Virus: Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
-
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW (2002) Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 29: 38–43. - PubMed
-
- Donegan E, Stuart M, Niland JC, Sacks HS, Azen SP, et al. (1990) Infection with human immunodeficiency virus type 1 (HIV-1) among recipients of antibody-positive blood donations. Ann Intern Med 113: 733–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UO1-HD-32632/HD/NICHD NIH HHS/United States
- UO1-AI-34994/AI/NIAID NIH HHS/United States
- UO1-AI-34989/AI/NIAID NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- UO1-AI-35004/AI/NIAID NIH HHS/United States
- R01 AI043894/AI/NIAID NIH HHS/United States
- UO1-AI-34993/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- R21 AI070740/AI/NIAID NIH HHS/United States
- UO1-AI-42590/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- UO1-AI-31834/AI/NIAID NIH HHS/United States
- R01 NS099029/NS/NINDS NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- AI070740/AI/NIAID NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

